Summary
The CsPbI3 inorganic perovskite is a potential candidate for fabricating long-term operational photovoltaic devices owing to its intrinsic superb thermal stability. However, the carbon-based CsPbI3 perovskite solar cells (C-PSCs) without hole transport material (HTM) are currently disadvantaged by their relatively low power conversion efficiency resulting from the poor grain quality and mismatched energy band levels of the as-made CsPbI3 films. Herein we demonstrate that by doping Na into the CsPbI3 lattice, the grain quality is significantly improved with low defect density, and also, the energy band levels are better matched to the contact electrodes, affording a higher built-in potential. Consequently, the Voc of the C-PSCs is drastically increased from 0.77 to 0.92 V, and the efficiency from 8.6% to 10.7%, a record value for the CsPbI3 PSCs without HTM. Moreover, the non-encapsulated device showed virtually no performance degradation after 70 days of storage in air atmosphere.
Subject Areas: Energy Sustainability, Materials Characterization, Energy Materials
Graphical Abstract
Highlights
-
•
Na doping improves the morphology and enhances the crystalline quality of CsPbI3 film
-
•
All energy band levels of Cs0.95Na0.05PbI3 are lifted up to match contact electrodes
-
•
Na-doped C-PSC achieves a PCE of 10.7%, a record value of the CsPbI3 PSC without HTM
-
•
The non-encapsulated Na-doped C-PSCs exhibit excellent stability in air
Energy Sustainability; Materials Characterization; Energy Materials
Introduction
Organic-inorganic hybrid perovskite solar cells (PSCs) have attracted tremendous attention for their rapidly rising power conversion efficiency (PCE), currently reaching over 23%, achieved by solution-based techniques (Kojima et al., 2009, Lee et al., 2012, Mei et al., 2014, Yang et al., 2017). However, the organic ions in the organic-inorganic hybrid perovskites can easily escape from lattice under thermal stress, which restricts their long-term practical application (Wang et al., 2016, Park et al., 2016, Kim et al., 2016, Manser et al., 2016). In this regard, inorganic perovskites appear to be highly promising light absorbers owing to their good thermal stability (Duan et al., 2018, Yang et al., 2018). Among various inorganic perovskites, CsPbI3 perovskite is the most suitable one because of its appropriate band gap (1.73 eV) for photovoltaic (PV) applications (Eperon et al., 2015, Frolova et al., 2016, Sanehira et al., 2017, Wang et al., 2018a, Wang et al., 2018c).
Other than the potential problems of perovskites as an active layer material in PSCs, the adoption of organic hole transport materials (HTMs) may also limit the stability of PSCs because they are commonly thermally unstable and susceptible to ion migration and metal electrode corrosion eventually causing device degradation (Swarnkar et al., 2016, Eperon et al., 2015, Luo et al., 2016, Liang et al., 2017b). To tackle this issue, proposal has been put forward to replace the organic HTM and the metal electrode with a carbon electrode, which for the past few years has proved to be an effective approach (Chen and Yang, 2017). Some works on carbon-based HTM-free PSCs (C-PSCs) have employed CsPbI3 as a light absorber, and in our own work, a PCE of 9.5% has been achieved previously (Xiang et al., 2018). However, such a PCE is far lower than those achieved by the C-PSCs based on organic-inorganic hybrid perovskites. According to the previous works, the low PCE can be mainly attributable to the low obtainable open-circuit voltage (Voc) (Ball et al., 2013, Han et al., 2015, Zhang et al., 2017, Liang et al., 2017a), i.e., below 0.8 V. The low Voc should originate from the low grain quality of CsPbI3 film or the mismatched energy band levels at charge collection interface (Ahmad et al., 2017, Eperon et al., 2015, Zhang et al., 2018, Chen et al., 2018, Sanehira et al., 2017).
Herein, we address the above issues by doping the CsPbI3 inorganic perovskite at A site with Na element. It is found that the Na doping not only improved the morphology of CsPbI3 film but also significantly enhanced the grain quality, thus reducing the defect density. Furthermore, the Na doping offers a good handle to adjust the energy band levels of CsPbI3 film, raising the built-in potential and Voc. As a result, C-PSCs based on the Na-doped CsPbI3 film achieved a PCE as high as 10.7% with a Voc of 0.92 V, which are considerably higher than those obtained by the C-PSCs based on pure CsPbI3 film (PCE = 8.6%, Voc = 0.77 V). Besides, the non-encapsulated C-PSCs based on the Na-doped CsPbI3 film exhibit almost no PCE degradation after 70 days of storage in a dry air atmosphere.
The whole procedure of C-PSC fabrication, including CsPbI3 deposition, was conducted in a dry air atmosphere (humidity∼10%–20%). The CsPbI3 films were deposited on TiO2 mesoporous scaffolds via a one-step spin-coating method with the precursor solution containing ∼1 M N,N-Dimethylformamide (DMF)·HI·PbI2, (1-x) M CsI, and x M NaI, followed by heating at 200°C to obtain black CsPbI3 perovskite films.
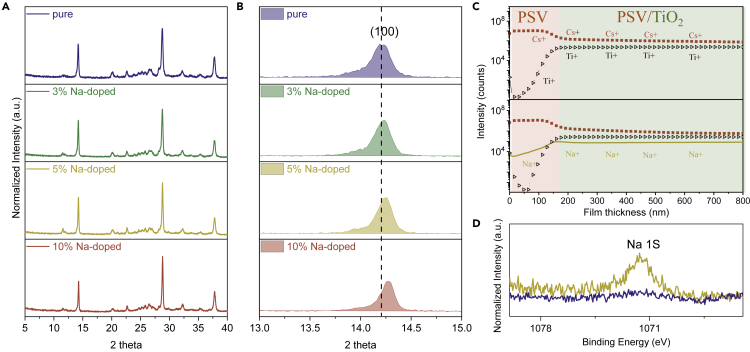
X-ray diffraction (XRD) patterns of the perovskite films with different Na doping concentrations are shown in Figure 1A. As depicted in Figures 1A and 1B, with Na doping content increasing, the (100) peak shifts to a higher 2θ, correlating to the lattice contraction. Therefore Na ions should have partially replaced Cs ions in the CsPbI3 lattice because Na atoms (1.02Å) are significantly smaller than Cs atoms (1.67Å). Moreover, as depicted in Figure S1, the films after storage for 7 days show almost the same XRD patterns as the corresponding as-prepared films, suggesting high phase stability. Secondary ion mass spectroscopy was further employed to study the composition difference between CsPbI3 and Cs0.95Na0.05PbI3 films. The depth profiling in Figures 1C and S2 shows that Na ions have been doped into the CsPbI3 film, which distribute throughout the whole film. As a side note, Na atom concentration in the capping layer is slightly lower than that in the mesoporous layer. This could be interpreted as follows. The tolerance factor of CsPbI3 perovskite is small (t = 0.81), and it would be further lowered after the substitution of Cs with Na atoms, which would reduce the structural stability. To make the structure stable, Na atoms might thermodynamically tend to stay in the mesoporous TiO2 scaffold, because the nanopores in the scaffold could confine grain dimensions to nanosize (Choi et al., 2013) and there may be lattice strain at the CsPbI3/TiO2 interface, which both would help to stabilize the perovskite structure. Similar phenomenon has been reported for organic-inorganic perovskites when the ions with a large size mismatch degree were used as dopants (Qiao et al., 2018). To identify the chemical states of CsPbI3 and Cs0.95Na0.05PbI3 films, X-ray photoelectron spectra (XPS) have also been recorded. The high-resolution XPS spectra for various elements (Na 1s, Cs 3d, Pb 4f and I 3d) are shown in Figures 1D and S3. Notably, a uniform shift in the peak positions of Cs 3d and Pb 4f to a lower binding energy is observed for Cs0.95Na0.05PbI3 film, whereas the peak position of I 3d shifts to a higher binding energy. These shifts should be correlated to the Na doping because the smaller Na atoms would cause the volume contraction of the BX6 (B = Pb or Mn; X = I or Br) octahedral and hence lead to the changes in chemical bonding (Zou et al., 2017, Li et al., 2016).
Figure 1.
Composition Characterizations of Cs1-xNaxPbI3 (0 ≤ x ≤ 0.1) Perovskite Films
(A) XRD patterns.
(B) Magnification of the (100) peak to show the peak shifts in the Na-doped film.
(C) Secondary ion mass spectroscopy depth profile for Cs, Pb, I, and Na elements of CsPbI3 and Cs0.95Na0.05PbI3 films.
(D) High-resolution XPS spectra of CsPbI3 and Cs0.95Na0.05PbI3 films.
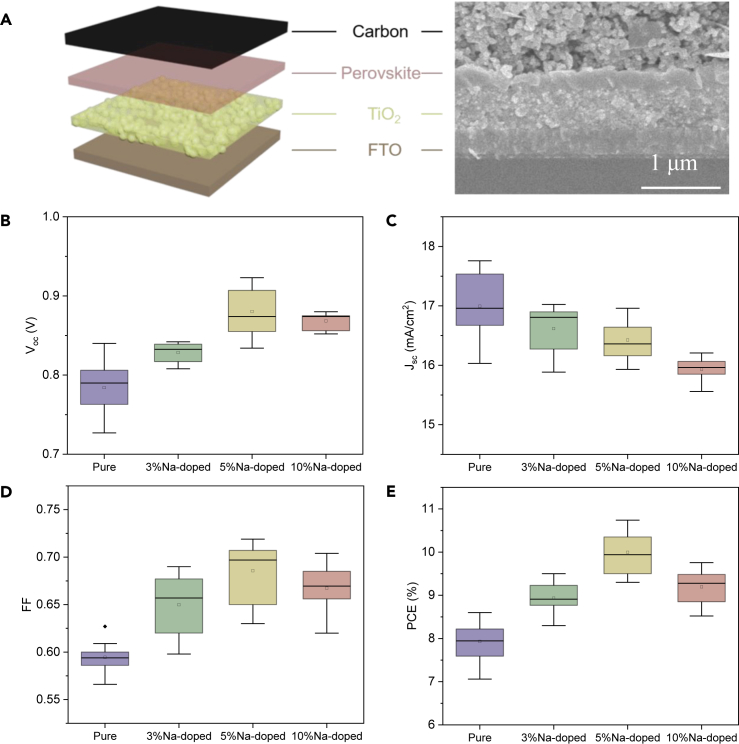
To evaluate PV performance, paintable C-PSCs (as illustrated in Figure 2A) were fabricated by directly painting a commercial carbon paste on the CsPbI3 and Cs0.95Na0.05PbI3 films, followed by annealing at 120°C for 30 min (Chen et al., 2015, Chen et al., 2016). The cross-sectional scanning electron microscopic (SEM) image of the C-PSCs in Figure 2A indicates an intimate contact at the CsPbI3/carbon interface.
Figure 2.
Device Structure and Photovoltaic Performance of CsPbI3 and Cs1-xNaxPbI3 (0 ≤ x ≤ 0.1) C-PSCs
(A) Scheme and cross-sectional SEM image of CsPbI3 C-PSCs.
(B–E) Statistical box chart of (B) Voc, (C) Jsc, (D) FF, and (E) PCE of CsPbI3 and Na-doped CsPbI3 C-PSCs.
The average PV parameters obtained from the CsPbI3 and Cs1-xNaxPbI3 (0 ≤ x ≤ 0.1) cells are shown in Figures 2B–2E. The average open-circuit voltage (Voc) increases progressively with Na incorporation, from 0.78 ± 0.02 V for x = 0 to 0.88 ± 0.02 V for x = 0.05, and then slightly declines to 0.86 ± 0.01 V for x = 0.10. With Na doping concentration increasing, the short-circuit current density (Jsc) decreases gradually, from 17.0 ± 0.53 mA/cm2 for x = 0 to 15.9 ± 0.1 mA/cm2 for x = 0.10. Similar with Voc, the fill factor (FF) also gradually increases as x increases from 0 to 0.05, and then reduces as x further increases to 0.10, with 5% Na (FF = 0.68 ± 0.02) outstanding among the others. As a result, the PCE increases from 7.9% ± 0.3% for the pristine CsPbI3 device to 10.0% ± 0.4% for the Cs0.95Na0.05PbI3 one. Interestingly, even for the perovskites containing 10% Na, the PV performance is still superior to the pure CsPbI3, with an average PCE of 9.19% ± 0.3%.
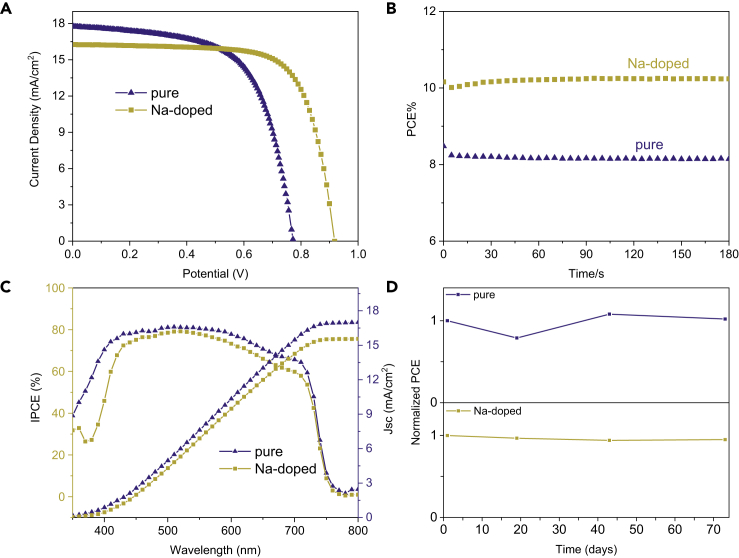
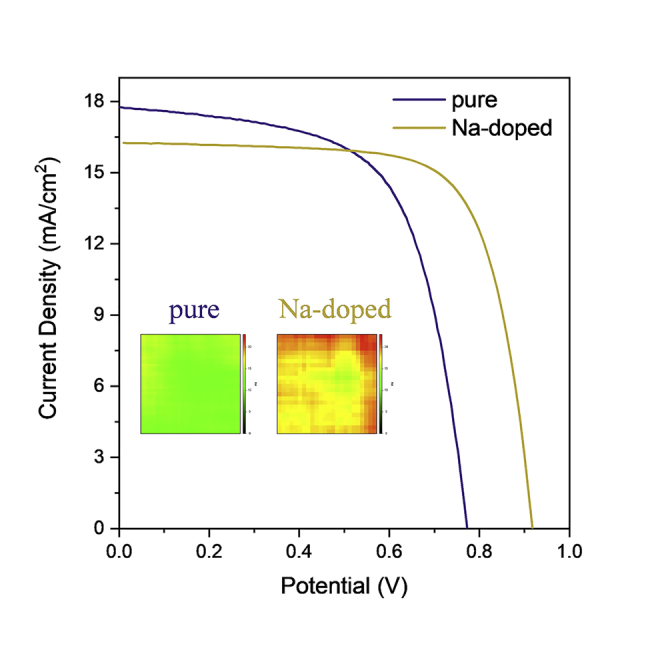
The current density versus voltage (J-V) curves of the champion CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs devices are presented in Figure 3A. The champion CsPbI3 C-PSCs obtain a Voc of 0.77 V, Jsc of 17.76 mA/cm2, and FF of 0.627, which leads to a PCE of 8.6%, whereas the champion Cs0.95Na0.05PbI3 device shows a Voc of 0.92 V, Jsc of 16.5mA/cm2, and FF of 0.703, which generates a PCE of 10.7%, a new record PCE for HTM-free CsPbI3 PSCs (Liang et al., 2017a, Xiang et al., 2018). It can be clearly seen that the large increase in PCE mainly benefits from the obvious improved Voc and FF, which would be further discussed below.
Figure 3.
Champion Photovoltaic Performance of CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs
(A–D) (A) J-V curves, (B) stabilized power output, (C) IPCE spectra and integrated Jsc, and (D) stability results of CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs that were stored in dry air atmosphere (20°C –35°C and 10%–20% humidity). All devices used for the performance and stability testing were non-encapsulated.
Figure 3B displays the plots of the PCEs that are obtained at the voltage close to the maximum power output point as a function of time, indicating that under constant illumination the steady-state PCEs of the CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs are about 10.23% and 8.18%, respectively. In Figure S4, the J-V curves obtained from different scan directions show that both C-PSCs show similar hysteresis. The incident photon-to-electron conversion efficiency (IPCE) spectra (Figure 3C) are further taken for illustrating the Jsc decrease of the doped devices. Compared with Cs0.95Na0.05PbI3 C-PSCs, CsPbI3 C-PSCs show higher IPCE values in the whole wavelength, generating the integrated Jsc of 17.0 mA/cm2 versus 15.55 mA/cm2, which is well consistent with the Jsc extracted from the J-V curves. The cross-sectional SEM images in Figure S6A indicate that the film thickness of the two perovskite layers are almost the same (230 nm). Thus the lower absorption coefficient should be responsible for the decreased Jsc of Na-doped C-PSCs, as proved with the ultraviolet-visible (UV-vis) spectra in Figures 4C and S9.
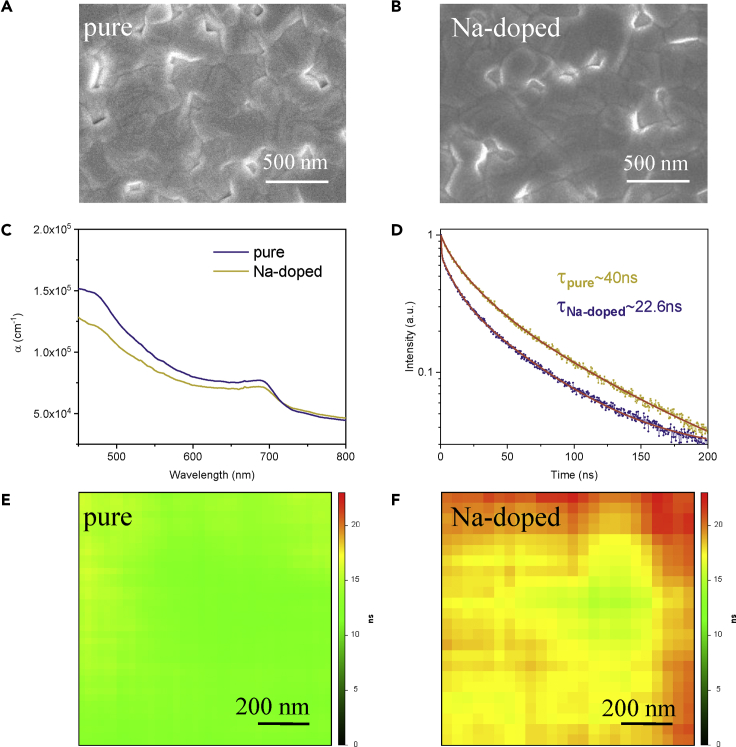
Figure 4.
Morphology and Optoelectronic Properties of CsPbI3 and Cs0.95Na0.05PbI3 Films
(A and B) Top-view SEM images of (A) CsPbI3 and (B) Cs0.95Na0.05PbI3 films.
(C–F) (C) Absorption coefficient (α) versus wavelength curves and (D) TRPL of CsPbI3 and Cs0.95Na0.05PbI3 films. Fluorescence lifetime mapping images of (E) CsPbI3 and (F) Cs0.95Na0.05PbI3 films.
The long-term stability of CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs was also tested (Figure 3D) with the non-encapsulated devices stored in dry air atmosphere (20°C–35°C and 10%–20% humidity). Promisingly, both C-PSCs show excellent stability and almost no PCE degradation is detected after storage for more than 70 days. The photostability measurement of the devices under continuous one-sun illumination (100 mW/cm−2) was also conducted in ambient atmosphere (20°C and 40% humidity). As shown in Figure S5, Cs0.95Na0.05PbI3 device demonstrates a higher photostability than the CsPbI3 one and 80% of the initial PCE has been retained after degradation for 24 h.
To get insights into the performance difference between the two C-PSCs, more characterizations on the perovskite films and C-PSCs were conducted. Top-view SEM images of CsPbI3 and Cs0.95Na0.05PbI3 films in Figures 4A and 4B show that after Na doping, the film surface becomes slightly smoother and the grain size becomes slightly larger, with the average size increasing from ∼243 nm to around 288 nm (as shown in Figure S7). As further confirmed by atomic force microscopic images in Figure S6B, the roughness value (Ra) decreases from 19.9 to 18.3 nm after Na doping. However, such small improvement in film morphology could not well account for the PV performance difference.
Absorption coefficient versus wavelength curves of TiO2/CsPbI3 and TiO2/Cs0.95Na0.05PbI3 thin films have been further measured. As shown in Figure 4C, the absorption coefficient decreases after Na doping, which well explains the decreased Jsc of the Na-doped devices. Steady-state photoluminescence (PL) and time-resolved PL (TRPL) spectra were further employed to evaluate the grain quality of CsPbI3 and Cs0.95Na0.05PbI3 films. As shown in Figure S8, the steady-state PL peaks of the two films appear at a very close wavelength, which is consistent with the UV-vis spectra in Figure S9. As shown in Figures S9C and S9D, the band gaps of CsPbI3 and Cs0.95Na0.05PbI3 are measured to be 1.67 and 1.68 eV, respectively. As clearly seen, the PL intensity of the Cs0.95Na0.05PbI3 film is significantly higher than that of the CsPbI3 film, suggesting a higher grain quality with less defects (Vorpahl et al., 2015, Tan et al., 2017, Pan et al., 2018). TRPL spectra of the bare perovskite films on glass substrate in Figure 4D show an obviously slower quenching rate after Na doping. After fitting, the average PL lifetime is calculated to be about 40 ns for the Cs0.95Na0.05PbI3 film, almost two times longer than that of the CsPbI3 film, well confirming the lower defect density induced by Na doping for suppressing charge recombination. To more vividly depict PL lifetime difference, the PL lifetimes throughout the perovskite films were also mapped. As shown in Figures 4E and 4F, the PL lifetime of the CsPbI3 film is mainly around 10–15 ns, as highlighted by the light green color, whereas most of the area on the Cs0.95Na0.05PbI3 film shows a PL lifetime of over 20 ns, as depicted by orange color. Therefore steady-state PL, TRPL, and PL lifetime mapping all confirm a higher grain quality with lower defect density after Na doping.
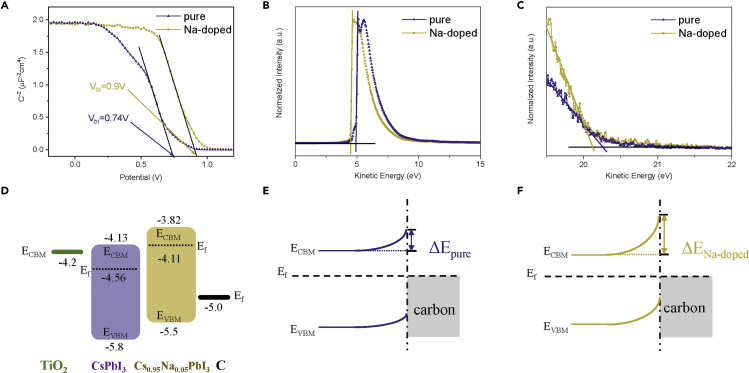
Mott-Schottky analyses of CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs were carried out to get built-in potential (Vbi), which is related to the Voc of C-PSCs (Laban and Etgar, 2013, Almora et al., 2016). As shown in Figure 5A, the Vbi can be obtained by using the intercept of the linear regime with the x axis of the Mott-Schottky plot (Almora et al., 2016). The Vbi for the CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs are about 0.74 V and 0.90 V, respectively. Such difference in Vbi is in good agreement with the Voc values obtained from the J-V curves in Figure 2B. The larger Vbi would assist in more efficient charge separation and collection, which is favorable for achieving higher Voc and FF (Wang et al., 2018b). Therefore the increase in Vbi should be an important cause for the performance improvement.
Figure 5.
Junction Properties and Carrier Behaviors in C-PSCs
(A–C) (A) Mott-Schottky analysis at 10 kHz for CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs. UV photoelectron spectroscopy of CsPbI3 and Cs0.95Na0.05PbI3 films: (B) cutoff region and (C) valence band edge region.
(D–F) (D) Energy level diagrams of CsPbI3 and Cs0.95Na0.05PbI3 C-PSCs, which were obtained from the UV photoemission spectroscopy results. Energy level diagram of the (E) CsPbI3/carbon and (F) Cs0.95Na0.05PbI3/carbon interfaces. EVBM represents the energy level of valence band maximum, ECBM represents the energy level of conduction band minimum, and Ef represents the Fermi level.
As well known, the difference in Vbi is usually reflected from the change in energy band levels (Wang et al., 2015). The energy band levels of CsPbI3 and Cs0.95Na0.05PbI3 films were characterized by the combination of UV photoelectron spectroscopy (Figures 5B and 5C) and UV-vis spectra (Figure S9). The final energy band levels of CsPbI3 and Cs0.95Na0.05PbI3 are depicted in Figure 5D. It can be indicated that both perovskite films are n-type semiconductors. Compared with CsPbI3 film, all energy band levels of Cs0.95Na0.05PbI3 film are obviously lifted, especially for Ef. The higher ECBM may be favorable for electron injection from Cs0.95Na0.05PbI3 to TiO2. The lifting of the Ef would induce a larger upward band bending at the Cs0.95Na0.05PbI3/carbon interface as depicted in Figures 5E and 5F, which would promote hole transfer. The obtained Vbi from the Mott-Schottky analysis may predominantly form at perovskite/carbon interface because the potential drop at the n-n junction of TiO2/perovskite interface should be negligible (Jiang et al., 2015, Guerrero et al., 2014).
According to intensity-modulated photocurrent/photovoltage spectroscopy results, the relationship of light intensity (I0) with electron diffusion coefficients (Dn) and electron lifetime (τn) could be obtained, as shown in Figure S10. Based on the equation Ln=(Dnτn)1/2, diffusion length (Ln) could be calculated (Zhu et al., 2007, Zhao and Zhu, 2013). As depicted in Figure S10C, the Ln of Cs0.95Na0.05PbI3 C-PSCs is obviously larger than that of CsPbI3 C-PSCs, which further confirms the lower charge recombination loss and more efficient charge collection.
In conclusion, we have significantly increased the Voc and PCE of CsPbI3 C-PSCs by doping Na into the CsPbI3 lattice. The resulting morphology shows a slight difference between CsPbI3 and Cs0.95Na0.05PbI3 films, but the grain quality was significantly improved with a lower defect density for the doped sample. Spectroscopic measurements showed that all energy band levels (EVBM, ECBM, and Ef) were lifted up after Na doping, which afforded a better match to the contact electrode and hence supported a larger Vbi for obtaining a higher Voc (0.92 V) for C-PSCs. As a result, the Cs0.95Na0.05PbI3 C-PSCs achieved a PCE as high as 10.7%, which is a new record value for the α-CsPbI3 PSCs without HTM. More promisingly, the non-encapsulated Cs0.95Na0.05PbI3 C-PSCs exhibited almost no performance degradation after 70-day storage in a dry air atmosphere. Therefore Na doping is an effective way to fabricate CsPbI3 C-PSCs with high performance and high stability.
Limitations of the Study
Organic-inorganic hybrid PSCs have attracted great interest because of their rapidly rising PCE. However, the volatility of the organic cations in these materials restricts long-term practical application. The CsPbI3 inorganic perovskite with intrinsic superb thermal stability is a potential candidate to fabricate prolonged stable operational PV devices. However, the efficiency of carbon-based PSCs (C-PSCs) without HTM, the most promising commercial PSCs, is relatively low due to the poor crystalline quality and mismatched energy band levels of CsPbI3. Herein, by doping Na into the CsPbI3 lattice, the PCE of C-PSCs is largely improved especially the open circuit potential. The important achievements are as follows:
-
(1)
The Na doping not only improves the morphology of CsPbI3 film but also significantly enhances crystalline quality to reduced defect density.
-
(2)
All energy band levels of CsPbI3 are lifted up to match contact electrodes after Na doping, which helps to support a much higher built-in potential for obtaining an increased Voc from 0.77 to 0.92 V.
-
(3)
Na-doped C-PSCs achieve an efficiency of 10.7%, a new record value of the CsPbI3 PSCs without HTM.
-
(4)
The non-encapsulated Na-doped C-PSCs exhibit almost no performance degradation after 70 days of storage in air atmosphere.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is financially supported by the Young Talent of “Zhuoyue” Program of Beihang University, the National Natural Science Foundation of China (21875013 and 21603010), and the Beijing Natural Science Foundation (No. 2182031). The authors would like to thank Senior Engineer Yan Guan in Peking University Analytical Instrumentation Center for the fluorescence lifetime mapping image supports. Shenzhen Peacock Plan (KQTD2016053015544057), the HK-RGC General Research Funds (GRF Nos. 16312216) and the HK Innovation and Technology Fund (GHP/079/17SZ).
Author Contributions
H.C. proposed the research and directed the study. S.X. prepared and characterized the perovskite films. Y.W. and J.L. helped with the device fabrication and characterization. W.L., H.L., and L.Z. helped analyzed the data. S.X., H.C., and S.Y. prepared the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.025.
Contributor Information
Shihe Yang, Email: chsyang@ust.hk.
Haining Chen, Email: chenhaining@buaa.edu.cn.
Supplemental Information
References
- Ahmad W., Khan J., Niu G., Tang J. Inorganic CsPbI3 perovskite-based solar cells: a choice for a tandem device. Solar RRL. 2017;1:1700048. [Google Scholar]
- Almora O., Aranda C., Mas-Marzá E., Garcia-Belmonte G. On Mott-Schottky analysis interpretation of capacitance measurements in organometal perovskite solar cells. Appl. Phys. Lett. 2016;109:173903. [Google Scholar]
- Ball J.M., Lee M.M., Hey A., Snaith H.J. Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ. Sci. 2013;6:1739–1743. [Google Scholar]
- Chen H., Wei Z., He H., Zheng X., Wong K.S., Yang S. Solvent engineering boosts the efficiency of paintable carbon-based perovskite solar cells to beyond 14% Adv. Energy Mater. 2016;6:1502087. [Google Scholar]
- Chen H., Wei Z., Zheng X., Yang S. A scalable electrodeposition route to the low-cost, versatile and controllable fabrication of perovskite solar cells. Nano Energy. 2015;15:216–226. [Google Scholar]
- Chen H., Xiang S., Li W., Liu H., Zhu L., Yang S. Inorganic perovskite solar cells: a rapidly growing field. Solar RRL. 2018;2:1700188. [Google Scholar]
- Chen H., Yang S. Carbon-based perovskite solar cells without hole transport materials: the front runner to the market? Adv. Mater. 2017;29:1603994. doi: 10.1002/adma.201603994. [DOI] [PubMed] [Google Scholar]
- Choi J.J., Yang X., Norman Z.M., Billinge S.J., Owen J.S. Structure of methylammonium lead iodide within mesoporous titanium dioxide: active material in high-performance perovskite solar cells. Nano Lett. 2013;14:127–133. doi: 10.1021/nl403514x. [DOI] [PubMed] [Google Scholar]
- Duan J., Zhao Y., He B., Tang Q. High-purity inorganic perovskite films for solar cells with 9.72% efficiency. Angew. Chem. Int. Ed. 2018;130:3849–3853. doi: 10.1002/anie.201800019. [DOI] [PubMed] [Google Scholar]
- Eperon G.E., Paterno G.M., Sutton R.J., Zampetti A., Haghighirad A.A., Cacialli F., Snaith H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A. 2015;3:19688–19695. [Google Scholar]
- Frolova L.A., Anokhin D.V., Piryazev A.A., Luchkin S.Y., Dremova N.N., Stevenson K.J., Troshin P.A. Highly efficient all-inorganic planar heterojunction perovskite solar cells produced by thermal coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2016;8:67–72. doi: 10.1021/acs.jpclett.6b02594. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Juarez-Perez E.J., Bisquert J., Mora-Sero I., Garcia-Belmonte G. Electrical field profile and doping in planar lead halide perovskite solar cells. Appl. Phys. Lett. 2014;105:133902. [Google Scholar]
- Han G.S., Chung H.S., Kim B.J., Kim D.H., Lee J.W., Swain B.S., Mahmood K., Yoo J.S., Park N.-G., Lee J.H. Retarding charge recombination in perovskite solar cells using ultrathin MgO-coated TiO 2 nanoparticulate films. J. Mater. Chem. A. 2015;3:9160–9164. [Google Scholar]
- Jiang C.-S., Yang M., Zhou Y., To B., Nanayakkara S.U., Luther J.M., Zhou W., Berry J.J., van de Lagemaat J., Padture N.P. Carrier separation and transport in perovskite solar cells studied by nanometre-scale profiling of electrical potential. Nat. Commun. 2015;6:8397. doi: 10.1038/ncomms9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Seo J.Y., Park N.G. Material and device stability in perovskite solar cells. ChemSusChem. 2016;9:2528–2540. doi: 10.1002/cssc.201600915. [DOI] [PubMed] [Google Scholar]
- Kojima A., Teshima K., Shirai Y., Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Laban W.A., Etgar L. Depleted hole conductor-free lead halide iodide heterojunction solar cells. Energy Environ. Sci. 2013;6:3249–3253. [Google Scholar]
- Lee M.M., Teuscher J., Miyasaka T., Murakami T.N., Snaith H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012;338:643–647. doi: 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- Li Z., Yang M., Park J.-S., Wei S.-H., Berry J.J., Zhu K. Stabilizing perovskite structures by tuning tolerance factor: formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016;28:284–292. [Google Scholar]
- Liang J., Wang C., Zhao P., Lu Z., Ma Y., Xu Z., Wang Y., Zhu H., Hu Y., Zhu G. Solution synthesis and phase control of inorganic perovskites for high-performance optoelectronic devices. Nanoscale. 2017;9:11841–11845. doi: 10.1039/c7nr03530f. [DOI] [PubMed] [Google Scholar]
- Liang J., Zhao P., Wang C., Wang Y., Hu Y., Zhu G., Ma L., Liu J., Jin Z. CsPb0. 9Sn0. 1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017;139:14009–14012. doi: 10.1021/jacs.7b07949. [DOI] [PubMed] [Google Scholar]
- Luo P., Xia W., Zhou S., Sun L., Cheng J., Xu C., Lu Y. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016;7:3603–3608. doi: 10.1021/acs.jpclett.6b01576. [DOI] [PubMed] [Google Scholar]
- Manser J.S., Saidaminov M.I., Christians J.A., Bakr O.M., Kamat P.V. Making and breaking of lead halide perovskites. Acc. Chem. Res. 2016;49:330–338. doi: 10.1021/acs.accounts.5b00455. [DOI] [PubMed] [Google Scholar]
- Mei A., Li X., Liu L., Ku Z., Liu T., Rong Y., Xu M., Hu M., Chen J., Yang Y. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science. 2014;345:295–298. doi: 10.1126/science.1254763. [DOI] [PubMed] [Google Scholar]
- Pan J., Shang Y., Yin J., de Bastiani M., Peng W., Dursun I., Sinatra L., el-Zohry A.M., Hedhili M.N., Emwas A.-H. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. J. Am. Chem. Soc. 2018;140:562–565. doi: 10.1021/jacs.7b10647. [DOI] [PubMed] [Google Scholar]
- Park N.-G., Grätzel M., Miyasaka T., Zhu K., Emery K. Towards stable and commercially available perovskite solar cells. Nat. Energy. 2016;1:16152. [Google Scholar]
- Qiao H.W., Yang S., Wang Y., Chen X., Wen T.Y., Tang L.J., Cheng Q., Hou Y., Zhao H., Yang H.G. A gradient heterostructure based on tolerance factor in high-performance perovskite solar cells with 0.84 fill factor. Adv. Mater. 2018;31:1804217. doi: 10.1002/adma.201804217. [DOI] [PubMed] [Google Scholar]
- Sanehira E.M., Marshall A.R., Christians J.A., Harvey S.P., Ciesielski P.N., Wheeler L.M., Schulz P., Lin L.Y., Beard M.C., Luther J.M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017;3:eaao4204. doi: 10.1126/sciadv.aao4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarnkar A., Marshall A.R., Sanehira E.M., Chernomordik B.D., Moore D.T., Christians J.A., Chakrabarti T., Luther J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science. 2016;354:92–95. doi: 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- Tan H., Jain A., Voznyy O., Lan X., de Arquer F.P.G., Fan J.Z., Quintero-Bermudez R., Yuan M., Zhang B., Zhao Y. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science. 2017;355:722–726. doi: 10.1126/science.aai9081. [DOI] [PubMed] [Google Scholar]
- Vorpahl S.M., Stranks S.D., Nagaoka H., Eperon G.E., Ziffer M.E., Snaith H.J., Ginger D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science. 2015;348:683–686. doi: 10.1126/science.aaa5333. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu X., Wang C., Xiao Z., Bi C., Shao Y., Huang J., Gao Y. Surface analytical investigation on organometal triiodide perovskite. J. Vac. Sci. Tech. B. 2015;33:032401. [Google Scholar]
- Wang D., Wright M., Elumalai N.K., Uddin A. Stability of perovskite solar cells. Solar Energy Mater. Sol. Cells. 2016;147:255–275. [Google Scholar]
- Wang P., Zhang X., Zhou Y., Jiang Q., Ye Q., Chu Z., Li X., Yang X., Yin Z., You J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018;9:2225. doi: 10.1038/s41467-018-04636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sakurai T., Wen W., Qi Y. Energy level alignment at interfaces in metal halide perovskite solar cells. Adv. Mater. Interfaces. 2018;5:1800260. [Google Scholar]
- Wang Y., Zhang T., Kan M., Zhao Y. Bifunctional stabilization of all-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 2018;140:12345–12348. doi: 10.1021/jacs.8b07927. [DOI] [PubMed] [Google Scholar]
- Xiang S., Fu Z., Li W., Wei Y., Liu J., Liu H., Zhu L., Zhang R., Chen H. Highly air-stable carbon-based α-CsPbI3 perovskite solar cells with a broadened optical spectrum. ACS Energy Lett. 2018;3:1824–1831. [Google Scholar]
- Yang F., Hirotani D., Kapil G., Kamarudin M.A., Ng C.H., Zhang Y., Shen Q., Hayase S. All-inorganic CsPb1-xGexI2Br perovskite with enhanced phase stability and photovoltaic performance. Angew. Chem. Int. Ed. 2018;57:12745–12749. doi: 10.1002/anie.201807270. [DOI] [PubMed] [Google Scholar]
- Yang W.S., Park B.-W., Jung E.H., Jeon N.J., Kim Y.C., Lee D.U., Shin S.S., Seo J., Kim E.K., Noh J.H. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science. 2017;356:1376–1379. doi: 10.1126/science.aan2301. [DOI] [PubMed] [Google Scholar]
- Zhang T., Dar M.I., Li G., Xu F., Guo N., Grätzel M., Zhao Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017;3:e1700841. doi: 10.1126/sciadv.1700841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang J., Phuyal D., Du J., Tian L., Öberg V.A., Johansson M.B., Cappel U.B., Karis O., Liu J. Inorganic CsPbI3 perovskite coating on PbS quantum dot for highly efficient and stable infrared light converting solar cells. Adv. Energy Mater. 2018;8:1702049. [Google Scholar]
- Zhao Y., Zhu K. Charge transport and recombination in perovskite (CH3NH3) PbI3 sensitized TiO2 solar cells. J. Phys. Chem. Lett. 2013;4:2880–2884. [Google Scholar]
- Zhu K., Neale N.R., Miedaner A., Frank A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007;7:69–74. doi: 10.1021/nl062000o. [DOI] [PubMed] [Google Scholar]
- Zou S., Liu Y., Li J., Liu C., Feng R., Jiang F., Li Y., Song J., Zeng H., Hong M. Stabilizing cesium lead halide perovskite lattice through Mn (II) substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017;139:11443–11450. doi: 10.1021/jacs.7b04000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.