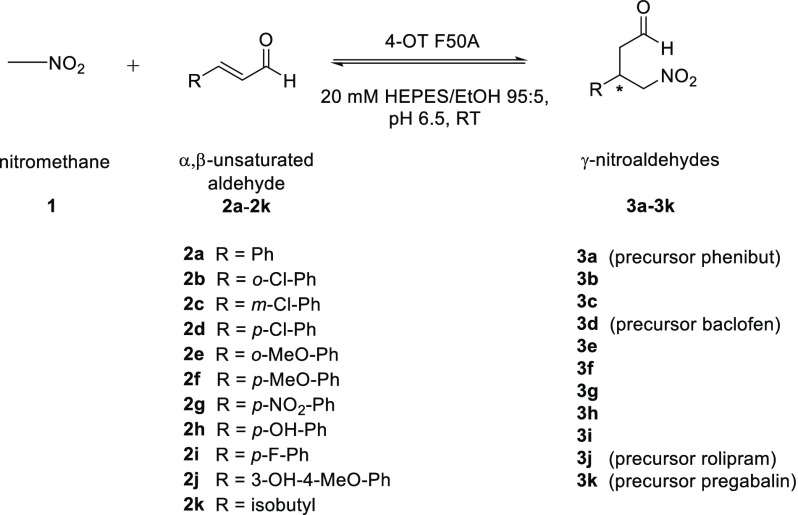
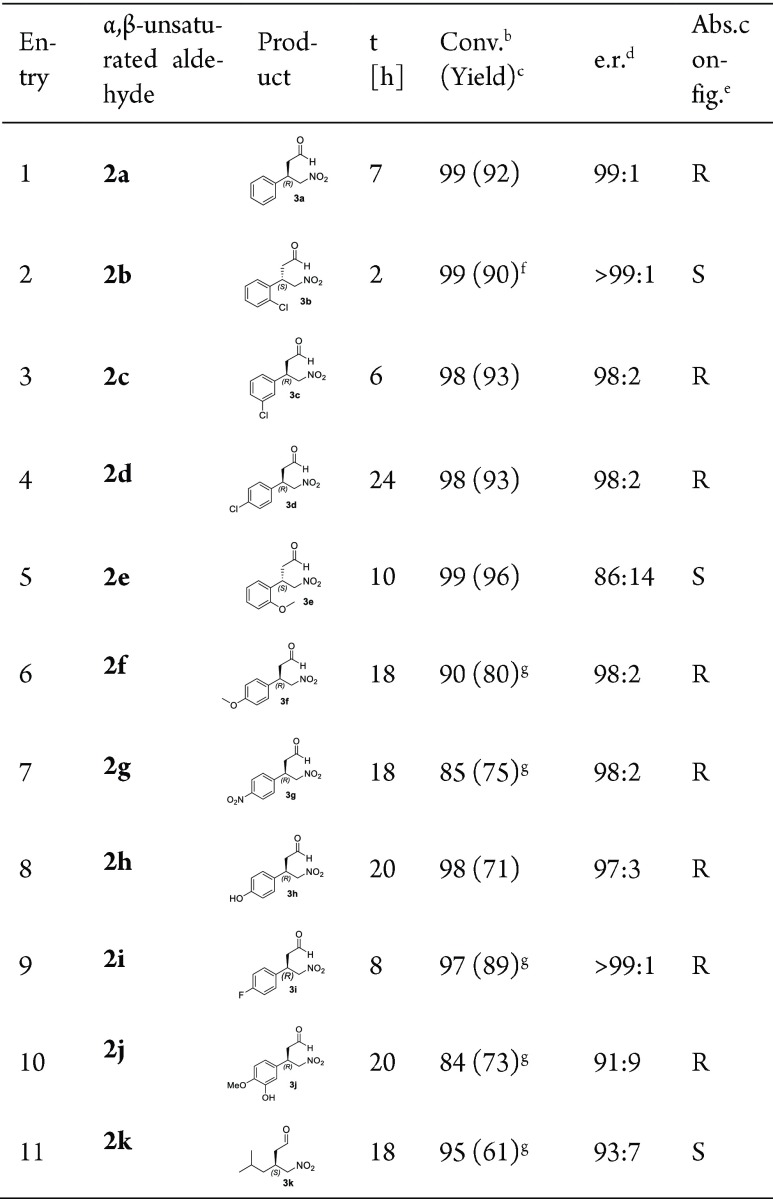
Table 1. 4-OT(F50A)-Catalyzed Nitromethane Addition to α,β-Unstaturated Aldehydes 2a–2k Using Optimized Reaction Conditionsa.
All the reactions were performed in buffer [20 mM HEPES/5% (v/v) ethanol] at pH 6.5 with 4-OT F50A (72 μM, except for 2g and 2i for which 36 μM enzyme was used), 1 (25 mM) and 2a–k (3 mM, except for 2g which was used at 2 mM).
Determined by 1H NMR analysis.
Isolated yield (%).
Determined by chiral HPLC or GC.
The absolute configuration was determined by comparison of chiral HPLC or GC data with those previously reported (see Supporting Information for details).
Apparent kinetic parameters determined with this substrate at a fixed nitromethane concentration of 25 mM: kcat = 0.05 (±0.002) s–1; Km = 367 (±37) μM.
Further purified using flash column chromatography.