Abstract
The versatility of mosquito species that spread emerging arthropod-borne viruses such as Zika has highlighted the urgent need to re-evaluate mosquito control standards. The prospect of using precise knowledge of the geographic distribution and vector status of local populations to guide targeted interventions has gained renewed attention, but the feasibility and utility of such an approach remain to be investigated. Using the example of mosquito management in the United States, we present ideas for designing, monitoring, and assessing precision vector control tailored to different environmental and epidemiological settings. We emphasize the technical adjustments that could be implemented in mosquito control districts to enable targeted control while strengthening traditional management.
Keywords: vector control, mosquito management, precision, arboviruses
The challenge of vector heterogeneity in mosquito control
Diverse mosquito control programs have recently been deployed in response to the rise in cases of several diseases spread to humans through the bite of infected mosquitoes [1–6]. These efforts have had significant impacts on disease transmission, but have also considerably affected the biology and distribution of mosquito populations and the pathogens they transmit. Therefore, effective mosquito-borne disease control now requires more specific approaches, with actions informed by locally relevant evidence and targeted to specific contexts.
Recent years have witnessed an increased availability of Geographic Information Systems (GIS) (see Glossary) and powerful sequencing and computing platforms enabling fine-scale analyses of environmental and molecular data. On the other hand, developing and applying novel technologies, especially electronic-based tools, is a growing trend in vector-borne disease management (Box 1). These technological outbreaks are renewing enthusiasm for control approaches that would be guided by knowledge of the environmental, entomological and epidemiological characteristics of the target area [7–10]. However an appraisal of the feasibility and benefits of specific interventions in well-defined operational settings is still lacking. Traditional Integrated Mosquito Control (IMC) as implemented in the United States for the surveillance of several mosquito-borne illnesses provides opportunities to re-evaluate vector control measures in light of recent technological advances. Here we re-examine the current protocols for mosquito-borne disease surveillance in the continental U.S. with the aim to address the feasibility and utility of precision mosquito management.
Box 1. Developing technologies and resources for mosquito control.
- Applying technologies to improve mosquito management at the individual, household or community level is a long-standing endeavor of vector control. Prospective mosquito control technologies now represent a multi-million dollar market featuring some of the largest companies in the world such as Google and Microsoft. The main trends include the following.
---Smart traps:
“smart traps” that can allow for rapid sorting and identification of mosquitoes or even sex-specific collections are being designed to circumvent some of the limitations of conventional traps such as the CDC light trap or ovitraps used for mosquito surveillance. The market of lethal traps that use CO2, octenol, heat, or light – or a combination of those – to lure mosquitoes in, then trap them in containers where they die is also growing exponentially.
---Aircrafts and drones:
sophisticated aircrafts have been used for decades for aerial adulticide and larvicide spraying around the world. Drones are being designed for a variety of mosquito control activities including seeking hard-to-find pockets of water, accessing difficult-to-reach areas, and achieving fast and efficient release of lab-reared mosquitoes in the wild.
---GIS-based equipment:
remote sensing from satellite images and super-sensitive radars can be used to map standing water and to detect the presence of adult mosquitoes at a fine scale, but the low resolution and the waiting time for data acquisition still limit the operational impact of these resources. A greater hope is placed upon the new generation of GIS-tools, consisting of user-friendly devices that are portable or incorporated in motorized equipment such as trucks.
---Genetic tools:
evidence from recent outbreaks suggests that the future of arboviral disease surveillance relies increasingly on hand-held DNA sequencing devices. Portable and affordable DNA sequencers are also expected to facilitate rapid and efficient diagnostic of population structure and pesticide resistance testing in mosquitoes.
---Other technologies:
other emerging tools such as portable acoustic devices used for killing mosquito larvae in water in the wild, cell phones used for mapping mosquito distribution or robots enabling rapid and efficient sorting and sexing of lab-reared adults for sterile male release programs may become key players in mosquito management in the near future.
---Citizen science and data science:
community engagement and accessible resources providing detailed information on local vector populations are a key component of mosquito management. Very encouraging examples of citizen science and data science programs for mosquito control include the NASA’s GLOBE Mosquito Habitat Mapper, The Global Mosquito Alert Consortium, VectorBase’s Population Biology (PopBio) resource v, and several success stories on public engagement in mosquito-borne disease management reported in Europe and in the U.S. [28,64].
Mosquito control in the continental U.S.
A recent report have indicated an alarming rise in the burden of vector-borne diseases in the U.S. over the past fifteen years [11]. Lyme-carrying vectors—ticks—are a constant public health concern, but the incidence of several diseases spread by mosquitoes is the most significant. Lack of vaccines for most mosquito-borne diseases therefore puts effective diagnosis, clinical management, and vector control methods at the forefront of management strategies for these diseases. Mosquito-borne diseases such as malaria and yellow fever have posed major challenges to public health over the last centuries in the continental U.S., particularly in the humid subtropical zone [12]. Since 1930, significant public interventions have been implemented to reduce exposure to mosquito vectors that occur in the US. Despite great strides such as malaria eradication in the 1950s, the arrival of West Nile virus in 1999 coupled with the recent geographic expansion of invasive Aedes mosquito species has dramatically increased the risk of several mosquito-borne illnesses across the U.S. [13–15]. Local and federal authorities have recently stepped up mosquito control effort in response to this epidemiological change.
More than 1,900 vector control organizations are responsible for designing and implementing early warning systems for several mosquito-borne diseases as well as integrated mosquito control within delimited geographic areas across the U.S. [16]. Mosquito control professionals perform year-round surveillance and deploy intervention measures when needed. Common methods used in IMC include public information and education, source reduction and habitat management, chemical control, biological control, and microbial control [10]. Larval control is done by removing standing water or by using biological or chemical larvicides to eliminate mosquito larvae. Community education programs consist of using several media platforms to provide information to the public on how to avoid mosquito bites and mosquito-borne illnesses. When surveillance activities show that adult mosquito populations are on the rise or that there is an increase in the spread of mosquito-borne viruses, professionals may decide to apply adulticides using backpack sprayers, trucks or airplanes [10]. To manage major mosquito-borne disease outbreaks (e.g., the 2016 arrival of Zika in Florida), local health departments and mosquito control districts (MCDs) partner with various agencies—including the Centers for Disease Control and prevention (CDC)—to implement more intense interventions such as massive insecticide spraying in addition to traditional IMC [17].
On the one hand, reduced financial resources deeply limit the competences of mosquito control districts as shown by a recent nation-wide survey [16]. Yet on the other hand, a significant number of novel technologies designed to improve vector control are reaching the market (Box 1). It is clear that controlling versatile mosquito species that spread emerging arthropod-borne viruses (arboviruses) in unexpected places does require more flexible interventions capable of determining, predicting and accounting for variability within and between pest populations. Therefore, examining how the technology can empower such type of interventions should be a key component in decision-making regarding the acquisition of novel technologies in MCDs..
Definition of precision mosquito control
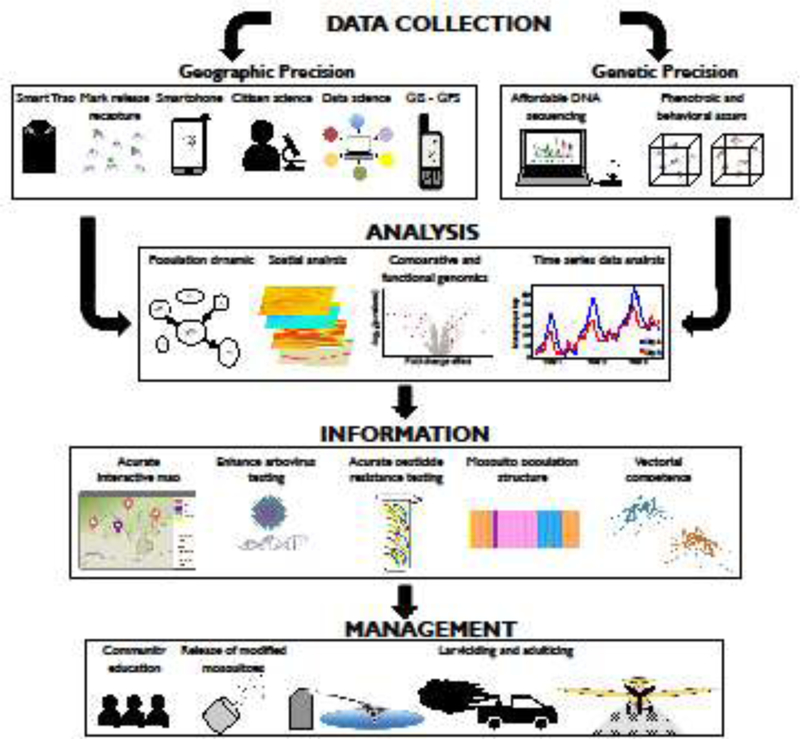
Implementing spatially targeted interventions is a long-standing idea in vector-borne disease control [18]. For instance, the prospect of using entomological indicators such as indoor resting densities to perform targeted malaria control has been around for two decades [19,20]. Targeted interventions may be more conceivable now than they were two decades ago due to considerable progresses in our understanding of mosquito ecology and genetics. However, questions remain about the feasibility and the capacity of such interventions to be efficiently embedded in integrated mosquito management. In this study, we have defined “precision mosquito control” in light of two related concepts: “precision medicine” and “precision public health”, which aim to promote intervention strategies that use technologies (e.g., genomics, spatial analysis, big data, etc.) to take individual- or population-level variations into account [21,22]. In the next sections, we have assessed the potential benefits of precision control by addressing the different competencies of integrated mosquito management whose outcomes can be improved with novel technologies (Table 1) (Figure 1). We have also emphasized some of the technical adjustments needed to make MCDs competent in implementing precise surveillance.
Table 1:
Core and supplemental competencies of traditional integrated mosquito management and some precision control methodologies that could be foreseeable in relatively well-equipped mosquito control districts. Other precision competencies that are not yet feasible due to knowledge gaps or technical difficulties are also suggested as future improvements, which will require long-term actions.
| Core competencies | |||||
|---|---|---|---|---|---|
| Traditional integrated mosquito management* | 1. Routine mosquito surveillance through standardized trapping and species identification | 2. Treatment decisions using surveillance data | 3. Larviciding, adulticiding or both | 4. Routine vector control activities | 5. Pesticide resistance testing |
| Precision mosquito control | • Smart traps • Interactive GIS maps • Time-series data collection • Spatial analysis • Citizen science • Data science • Mark-release recapture |
• Advanced pesticide resistance testing • Mosquito population size • Advanced arbovirus detection • Mosquito population structure |
• Targeting clusters of mosquito densities • Use of drones • Efficient chemical application • Resistance monitoring • Monitoring the impacts |
• Geographic precision • Targeted control • Accurate surveillance data |
• Unbiased phenotypic tests • Advanced molecular detection |
| Future improvements | • Population dynamics | • Mosquito fitness • Vector competence • Mosquito microbiome |
• Evaluation of precision control capabilities | • Insecticide resistance and mosquito fitness • Insecticide resistance and vector competence • Field trials |
|
| Supplemental competencies | |||||
| Traditional integrated mosquito management* | 6. Licenced pesticide application | 7. Vector control activities other than chemical control | 8. Community outreach and education campaigns regarding mosquito-borne diseases | 9. Regular communication with local health departments regarding surveillance and epidemiology | 10. Outreach with nearby vector control programs |
| Precision mosquito control | • Flexible regulation | • Geographic Precision | • Citizen science • Interactive GIS maps • Web developmennntnt |
• Data science • Interactive GIS maps • Web development |
• Data science • Citizen science • Flexible regulation |
Figure 1: Summary of some of the main features of precision mosquito control that could be implemented at the level of a mosquito control district.
Capacity strengthening in various aspects of geographic and genetic precision could empower mosquito control professionals to acquire fine-scale data on vector distribution and disease transmission. Several analytical procedures may be utilized to decipher the determinants of mosquito abundance, vectorial capacity and disease transmission within a designated geographic area. This information could then be used to inform targeted area-wide mosquito control measures.
Accounting for geographic and temporal heterogeneity
An important element of mosquito control consists of finding the vector populations and assessing the risk they represent for public health. Key components of alert systems that trigger interventions and guide the spatial prioritization of vector control during mosquito season in the U.S. include detecting geographic clusters of mosquito abundance or mosquito-borne disease cases in humans, and testing the prevalence of arboviruses in animal reservoir or in vector populations. Analysing spatial and temporal patterns of entomological and epidemiological indicators is therefore a cornerstone of risk assessment for arboviral disease control [10].
A central goal of research fields such as Geospatial Health or Spatial Epidemiology consists of using a Geographic Information System framework to model the relationships between the spatial distribution of human pathogens, their vectors, the prevalence of vector-borne diseases, and some environmental predictors. Although interest in these research fields has continued to grow in vector biology [7,23–27], the use of spatial distribution models in day-to-day vector control activities remains limited. Several obstacles including seasonal distribution and rapid range shifts make it difficult to reliably model the habitats and epidemiological significance of mosquito vectors that occur in the U.S. In particular, the spatial distribution and disease risk associated with invasive Aedes species that spread or disappear rapidly in different places are notoriously difficult to predict with a spatio-temporal resolution that would be relevant to MCDs. These limitations coupled with the significant level of skill required contribute to undermine the interest of mosquito control professionals for spatial distribution models as shown by recent surveys [16].
However, a new wave of GIS-based applications designed by MCDs, academic partners and private companies is rapidly emerging (Box 1). Some of these tools particularly suited for field operations and for engaging the public at the scale of a MCD. Notable examples include a series of user-friendly and accessible tools such as interactive maps used by mosquito control professionals to locate vector distribution and to inform the public in MCDs. GIS-equipped trucks are also enhancing the capacity to track larval habitats and to apply larvicides and adulticides exactly where needed. Finally, several citizen science projects that have been precious in identifying mosquito vectors and assessing vector-borne disease risk in some regions would have not been possible without the help of GIS tools enabling accurate geographic positioning and data management [28] (Box 1). In the long run, in addition to their critical role in field operations and outreach activities, novel GIS-based tools may empower MCDs to routinely collect fine-scale data that best reflect the fitness and epidemiological significance of local populations. Therefore, developing and applying tools enabling geographic precision within MCDs should be an integral part of mosquito-borne disease surveillance and would likely improve both standard management and the prospects of targeted control (see Outstanding questions) (Figure 1).
Precision genomics in mosquito control
High-quality genomic data on insect pests, human pathogens and microbiomes of disease vectors are rapidly accumulating i-iv. In addition, the increased availability of affordable and hand-held devices (e.g., Illumina MiSeq® and MinION®) allowing for point-of-need DNA sequencing makes us think that genomic expertise may be within the reach of some MCDs. However, despite a plethora of scientific publications in mosquito genomics, many conditions remain to be fulfilled to convert genomic knowledge into operationally viable tools for vector control. Genomic information can be helpful for at least three aspects relevant to mosquito-borne disease surveillance at the level of MCDs: pesticide resistance testing, arbovirus surveillance and improving the understanding of the factors underlying the vectorial capacity of mosquito populations in a given region (Figure 1, Table 1).
Pesticide resistance testing
Assessing the sensitivity of local populations is critical to predict the efficacy of chemical pesticides used in mosquito control and to understand and mitigate the epidemiological and operational implications of pesticide resistance [6,29]. For example, analysis revealed that aerial and truck sprays of several classes of insecticides had no or little effect on adult Aedes aegypti mosquito counts during the Zika outbreak in July, 2016, Miami-Dade County, Florida [17]. Such observations highlights the need to better understand the factors that are crucial to the success of chemical control. Pesticide resistance tests performed in MCDs consist of lab-based standard bioassays [16,30,31]. This phenotypic test aims to determine the proportion of individuals that can survive exposure to a standard dose of pesticide for a given period of time. In a few well-equipped MCDs, a Polymerase Chain Reaction (PCR)-based detection of mutations of the voltage gate sodium channel gene associated with increased resistance is occasionally performed in addition to bioassays (Table 1).
The mechanisms and consequences of insecticide resistance in insects are incredibly complex, and we may never have a complete understanding of the processes underlying the rapid adaptation of mosquito populations to chemical insecticides [32–35]. Pesticide exposure triggers a variety of adaptive changes, reflecting complex polygenic adaptation that would be best addressed with a combination of genomic tools, phenotypic and behavioural tests and field trials. One of the main challenges of insecticide resistance monitoring is developing molecular assays that may simultaneously screen favourable mutations at a large number of genetic loci associated with resistance. Recent genome-scale studies have pinpointed a significant number of genes involved in insecticide resistance that can be harnessed to develop multiplex polygenic tests in mosquitoes [36,37]. Such tests could be implemented using PCR or affordable next generation sequencing platforms accessible to MCDs and could be crucial to better characterize insecticide resistance and its operational and epidemiological consequences.
Enhancing arbovirus surveillance
Arbovirus surveillance requires special infrastructure and trained personnel, and only a few accredited laboratories have the capacity to perform arbovirus testing across the U.S. [38]. Traditionally, the presence of a list of known arboviruses is detected in animal reservoirs, humans hosts or mosquitoes using immunoglobulin tests, PCR or virus isolation tests [39]. Prior to arbovirus testing in mosquitoes, specimens have to be sorted and identified, which can be labor intensive and time consuming. Novel diagnostic tools for arbovirus detection in mosquitoes based on digital health systems and nanotechnology, honey-baited nucleic acid preservation cards or sugar bait stations, or high-throughput DNA sequencing are being developed to mitigate some of the limitations of current protocols [39–43].
Importantly, the rapid evolution of arboviruses as well as the emergence/re-emergence of new species highlights the need for advanced testing procedures that may provide a fine-scale description of genetic variation within the virus genome. Indeed, our understanding of the conditions that favor the emergence of new viral lineages responsible for outbreaks, as well as the drivers of their fitness and virulence remains limited [44–48]. Laboratory protocols are being developed to sequence RNA viruses directly from clinical samples in a way that is cheap, accurate, and scalable under resource limited conditions [49,50]. Interestingly, these protocols can be used with hand-held DNA sequencers and provide a potentially effective diagnostic tool for examining the genetic diversity of viral isolates in MCD/State laboratories. These advanced molecular tests will likely lay the foundation for a more precise detection of viral lineages and improve our understanding of the epidemiological relevance of genetic variation in arboviruses.
Deciphering the vectorial capacity of local mosquito populations
Within a mosquito vector species, vector competence for a given pathogen is limited to a fraction of individuals that ultimately contribute to disease transmission [51–53]. Identifying this fraction of population as well as the environmental and genetic factors that increase their fitness of in a given region would provide vital information for mosquito-borne disease surveillance and for designing targeted control. Unfortunately, despite significant research, the phenotypes and genes associated with mosquito fitness and its ability to transmit pathogens remain poorly understood. In theory, observational and experimental approaches based on comparative and functional genomics may help uncover some of the fitness traits and extrinsic factors that shape vectorial capacity of local populations [54–56]. However, the genetic architecture of vectorial capacity is extremely complex and it remains unclear whether state-of-the-art genomic studies can yield insights into what makes mosquito species efficient or prolific in terms of reproductive success of the vector populations in a given area. In addition, efficient genomic studies in mosquitoes are costly, require high levels of skills, and would be complicated to implement even in the best-equipped MCDs. Nevertheless, genomic techniques such as reduced representation sequencing [57] are cost-effective and are transferrable to MCD/State laboratories close to operational settings. Those may provide effective ways to address population subdivisions within a geographic region. Even though the underlying traits remain elusive, knowledge of local population structure is crucial to better understand the patterns of colonisation and the putative origin of invasive mosquito species.
In summary, genomic precision in mosquito control has an unfulfilled potential that should neither be underestimated nor overstated. Most variables that are vital for targeted control are underpinned by complex traits that are difficult to study even with powerful genomic analyses. Capacity building in genomics in MCDs could prioritize aspects that will be both helpful and feasible in MCD facilities in the near future such as rapid and efficient insecticide resistance testing, analysis of mosquito population structure and arbovirus testing (Table 1).
Monitoring the impacts of mosquito control
Ideally, monitoring the effects of human interventions on mosquito physiology, ecology, and vectorial capacity requires in-depth analyses of wild populations with multidisciplinary approaches (Table 1). Advanced molecular and geographic surveys may become increasingly feasible in well-equipped MCDs if capacity building leverages the widespread availability of precision technologies. One aspect that necessitates further scrutiny is the dynamic of vector populations. Variations in mosquito population size are challenging to quantify, and the effects of human interventions are difficult to disentangle from other natural drivers that modulate the presence and abundance of a species in a given environment.
Mosquito population size can be estimated via direct counts using traps, mark-release-recapture experiments or genetic tests. Combining improved trapping methods such as the use of smart traps, time-series data collections, spatial analysis, and effective citizen science and data science programs will likely contribute to more reliable evaluations of mosquito densities (Box 1). In principle, all these technical adjustments are accessible to normally equipped MCDs and would be particularly helpful in addressing the dynamics of invasive species with erratic natural cycles. Mark-release-recapture experiments are labour-intensive, but have been effectively used to estimate Aedes population size in small areas and may provide a valid alternative approach in some contexts [58]. Genetic tests aim to detect acute changes in population size due to variations in the number of individuals whose genetic material is passed onto the next generation. However, genome-scale studies have shown that the demographic changes possibly associated with vector control efforts are either too weak or undetectable at the genetic level in large mosquito populations, even with powerful sequencing technologies [36,59]. Therefore, it is unclear whether genetic analyses of population size, which are technically challenging, would be of any utility in MCDs. In the short term, robust estimates of mosquito densities may effectively guide decision-making and responses to alert protocols. Nonetheless, there is a crucial need to design other indicators that can be used in MCDs to monitor the long-term effects of area-wide control strategies on mosquito populations and disease risk.
The release of modified mosquitoes
One of the strategies currently being developed to control mosquito-borne diseases consists of releasing modified mosquitoes that are equipped to reduce the fertility of wild populations with whom they mate or to lower their ability to transmit pathogens [4,5,60–63]. Reducing mosquito populations or their epidemiological significance in a geographic area can be achieved via the release of males sterilized with radiation, males or females infected with a bacterium (Wolbachia), or genetically modified individuals.
A Wolbachia-based method is being tested in Fresno, California and in Miami, Florida to control Aedes aegypti populations. Wolbachia is not naturally present in wild Aedes aegypti and females lay eggs that do not hatch when they mate with males that have been artificially infected with the bacterium and released in nature. This mosquito control strategy is therefore sustained by recurrent releases of millions of Wolbachia-infected adult males, whose flight range is typically 30–100 meters, with the hope to reduce fertility and suppress naturally occurring populations within the precisely delimited geographical area. As a result, fine-scale analysis of geographic clusters of mosquito abundance in localized areas would be crucial to the effective deployment and monitoring of this method. Empowering MCDs to effectively perform spatial analyses would be vital to gain a precise idea about the optimal spatial extent, resolution and spacing needed for the releases.
Concluding remarks
The greatest successes achieved so far in controlling important vector-borne illnesses worldwide including the recent global reduction of malaria cases have been based on uniform deployment of conventional methods such as insecticide application or bed net distribution. A central question is to know if, given the complex challenge of emerging arthropod-borne diseases, surveillance programs can be effective and sustainable without a progressive incorporation of tools enabling precision management (see Outstanding Questions).
Integrated mosquito management programs are increasingly challenged to reduce costs, duration and environmental impacts, to engage the public and to comply with multiple corporate and public regulations. These requirements have increased the urgency to develop and apply novel technologies that may optimize surveillance and control (Box 1). Nonetheless, while applying technologies is gaining momentum—especially in wealthy MCDs—the capacity to form a decision support system that corresponds to inter- and intra-population variability remains weak. The potential benefits of new technologies in mosquito management are obvious, but their optimal application relies on capacity building in MCDs. Precision management in particular is hampered by our limited knowledge of components of vectorial capacity that could be used to target local populations as well as the significant cost, time and skills needed to implement complex analytical approaches. However, in addition to reinforcing the financial and technical capacity of MCDs, novel methodological frameworks and technologies should be incorporated in integrated vector management protocols to meet the ever-growing challenges due to emerging/re-emerging vector-borne illnesses. The technical capacity and the human and financial resources vary tremendously from one MCD to another. Therefore, it is difficult to precisely tell what technical adjustments could be feasible or should be prioritized to reinforce the competencies of MCD/State facilities. With this caveat in mind, we have indicated precision control methodologies whose implementation in relatively well-equipped MCDs could be foreseeable now or in the near future (Table 1). If some of the hurdles that undermine targeted control can be overcome, precision management may provide efficient tools to achieve effective vector-borne disease surveillance in contexts where higher standards of intervention are needed.
Highlights.
New strategies to tackle emerging arthropod-borne diseases are urgently needed; this will not only require new products and technologies, but also more precise and flexible management approaches.
The proliferation of advanced technologies in vector control lays the foundation for designing and monitoring specific mosquito surveillance programs that best suit the needs, the resources and the environmental and epidemiological characteristics of a target region.
Critical knowledge gaps and the limited resources of mosquito control organizations impede widespread application of precision management, but the feasibility of targeted measures is increasing, as well as the number of contexts where specific control is needed.
Acknowledgements
We are grateful for the insightful comments of two referees. CF and CK were supported by NIH grant 5R01AI113248-05 to Peter Atkinson.
Glossary
- Adulticide
a type of pesticide that may be used by a mosquito control program, a licensed pest control professional, or as a do-it-yourself application to kill adult mosquitoes.
- Citizen science
projects creating collaborations between professional scientists and the general public in order to expand opportunities for scientific data collection while raising awareness for research questions that affect people’s life.
- Data science
an interdisciplinary field, which combines several types of expertise at the intersection of the fields of statistics, information and computer science, to design and manage large databases.
- Geographic Information Systems (GIS)
a set of tools that enable acquiring, analysing and displaying geographically referenced information in the form of maps.
- Integrated Mosquito Control (IMC)
a term used to describe the comprehensive approach of managing mosquito populations that utilizes various techniques in order to reduce mosquito numbers and protect public health while minimizing the adverse environmental effects.
- Larvicide
a pesticide designed for killing the immature forms of an insect such as mosquito larvae and pupae.
- Mark-release-recapture experiments
a method used to estimate population size, which involves capturing, marking and releasing a sample of individuals in nature. Another sample of marked individuals are then recaptured and counted to estimate parameters such as life expectancy, flight distance, and dispersal and to infer population size.
- Mosquito Control District (MCD)
an independent special district charged with managing mosquito populations at levels that reduce the risk of mosquito-borne disease transmissions to humans and other vertebrates within a designated geographic area.
- Polygenic adaptation
a process in which a population adapts through small changes in allele frequencies at a large number of loci.
- Spatial analysis
a set of analytical techniques employed to determine the spatial distribution of a variable, the relationship between the spatial distribution of variables, and the association of the variables of an area using geographic information systems.
- Vector-borne disease
infections caused by pathogens transmitted by the bite of infected arthropod species—collectively called “vectors”—such as mosquitoes, ticks, triatomine bugs, sandflies, and blackflies.
- Vector control
a series of measures and methods used to reduce exposure to vector populations or to mitigate their ability to transmit pathogens.
- Vectorial capacity
a measurement of the efficiency of vector-borne disease transmission, which depends on multiple factors such as the incubation period of the pathogen, the survival rate of the vector, as well as other behavioural, environmental and ecological features.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Resources
References
- 1.Gubler DJ (2002) The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res 33, 330–342 [DOI] [PubMed] [Google Scholar]
- 2.Mayer SV et al. (2017) The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop 166, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould E et al. (2017) Emerging arboviruses: Why today? One Heal 4, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores HA and O’Neill SL (2018) Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol 16, 508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alphey L (2014) Genetic Control of Mosquitoes. Annu. Rev. Entomol 59, 205–224 [DOI] [PubMed] [Google Scholar]
- 6.Hemingway J (2017) The way forward for vector control. Science (80-. ) 358, 998–999 [DOI] [PubMed] [Google Scholar]
- 7.Clements ACA et al. (2013) Further shrinking the malaria map: How can geospatial science help to achieve malaria elimination? Lancet Infect. Dis 13, 709–718 [DOI] [PubMed] [Google Scholar]
- 8.Rinker DC et al. (2016) Disease vectors in the era of next generation sequencing. Genome Biol 17, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesolowski A et al. (2018) Mapping malaria by combining parasite genomic and epidemiologic data. BMC Biomed DOI: 10.1186/s12916-018-1181-9 [DOI] [PMC free article] [PubMed]
- 10.American Mosquito Control Association (2017) Best Practices for Integrated Mosquito Management: A Focused Update
- 11.Rosenberg R et al. (2018) Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67, 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Madriñán MJ and Turell M (2018) History of Mosquitoborne Diseases in the United States and Implications for New Pathogens. Emerg. Infect. Dis 24, 821–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JR (2001) The Control of Mosquito-Borne Diseases in New York City. J. Urban Heal. Bull. New York Acad. Med 78, 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams LL (1963) Malaria eradication in the United States. Am. J. Public Health Nations. Health 53, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell JR and Tabachnick WJ (2013) History of domestication and spread of Aedes aegypti - A Review. Mem Inst Oswaldo Cruz 108, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Association of County and City Health Officials (NACCHO) (2017) Mosquito Control Capabilities in the U.S
- 17.Stoddard PK (2018) Managing Aedes aegypti populations in the first Zika transmission zones in the continental United States. Acta Trop 187, 108–118 [DOI] [PubMed] [Google Scholar]
- 18.Carter R et al. (2000) Spatial targeting of interventions against malaria. Bull. World Health Organ 78, 1401–1411 [PMC free article] [PubMed] [Google Scholar]
- 19.Protopopoff N et al. (2008) Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am. J. Trop. Med. Hyg 79, 12–18 [PubMed] [Google Scholar]
- 20.Kamdem C et al. (2012) Spatially explicit analyses of anopheline mosquitoes indoor resting density: implications for malaria control. PLoS One 7, e31843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weeramanthri TS et al. (2018) Editorial : Precision Public Health. Front. Public Heal 6, 3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury MJ et al. (2017) From public health genomics to precision public health : a 20-year journey. Genet. Med 0, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Focks DA et al. (1999) The Use of Spatial Analysis in the Control and Risk Assessment of Vector-Borne Diseases. Am. Entomol 45, 173–183 [Google Scholar]
- 24.Palaniyandi M (2012) The role of remote sensing and GIS for spatial prediction of vector-borne diseases transmission: A systematic review. J. Vector Borne Dis 49, 197–204 [PubMed] [Google Scholar]
- 25.Eisen L and Lozano-Fuentes S (2009) Use of mapping and spatial and space-time modeling approaches in operational control of Aedes aegypti and dengue. PLoS Negl. Trop. Dis 3, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisen L and Eisen RJ (2011) Using Geographic Information Systems and Decision Support Systems for the Prediction, Prevention, and Control of Vector-Borne Diseases. Annu. Rev. Entomol 56, 41–61 [DOI] [PubMed] [Google Scholar]
- 27.Tjaden NB et al. (2018) Mosquito-Borne Diseases : Advances in Modelling Climate-Change Impacts. Trends Parasitol 34, 227–245 [DOI] [PubMed] [Google Scholar]
- 28.Jordan RC et al. (2017) Citizen Science as a Tool for Mosquito Control. J. Am. Mosq. Control Assoc 33, 241–245 [DOI] [PubMed] [Google Scholar]
- 29.WHO (2012) Global Plan for Insecticide Resistance Management in Malaria Vectors, World Health Organization. [Google Scholar]
- 30.Centers for Disease Control and Prevention (2012) Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay
- 31.Centers for Disease Control and Prevention (2016) Guideline for Aedes aegypti and Aedes albopictus Surveillance and Insecticide Resistance Testing in the United States
- 32.Liu N (2015) Insecticide Resistance in Mosquitoes : Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol 60, 537–59 [DOI] [PubMed] [Google Scholar]
- 33.Hemingway J and Ranson H (2000) Insecticide Ressistance In Insect Vectors Of Human Disease. Annu. Rev. Entomol 45, 371–391 [DOI] [PubMed] [Google Scholar]
- 34.ffrench-Constant RH (2013) The Molecular Genetics of Insecticide Resistance. Genetics 194, 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatton ML et al. (2013) The importance of moquito behavioural adaptations to malaria control in Africa. Evolution 67, 1218–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles A et al. (2017) Genetic diversity of the African malaria vector anopheles gambiae. Nature 552, 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faucon F et al. (2015) Unravelling genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res 25, 1347–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadler JL et al. (2015) Assessment of arbovirus surveillance 13 years after introduction of West Nile virus, United States. Emerg. Infect. Dis 21, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramírez AL et al. (2018) Searching for the proverbial needle in a haystack: Advances in mosquito-borne arbovirus surveillance. Parasites and Vectors 11, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batovska J et al. (2018) Effective mosquito and arbovirus surveillance using metabarcoding. Mol. Ecol. Resour 18, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batovska J et al. (2017) Metagenomic arbovirus detection using MinION nanopore sequencing. J. Virol. Methods 249, 79–84 [DOI] [PubMed] [Google Scholar]
- 42.Coffey LL et al. (2014) Enhanced arbovirus surveillance with deep sequencing: identification of novel rhabdoviruses and bunyaviruses in Australian mosquitoes. Virology 448, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell JA et al. (2018) Unbiased Strain-Typing of Arbovirus Directly from Mosquitoes Using Nanopore Sequencing: A Field-forward Biosurveillance Protocol. Sci. Rep 8, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grubaugh ND et al. (2016) Genetic Drift during Systemic Arbovirus Infection of Mosquito Vectors Leads to Decreased Relative Fitness during Host Switching. Cell Host Microbe 19, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grubaugh ND et al. (2017) Mosquitoes Transmit Unique West Nile Virus Populations during Each Feeding Episode. Cell Rep 19, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebel GD (2017) Promiscuous viruses—how do viruses survive multiple unrelated hosts? Curr. Opin. Virol 23, 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weger-Lucarelli J et al. (2018) Using barcoded Zika virus to assess virus population structure in vitro and in Aedes aegypti mosquitoes. Virology 521, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forrester NL et al. (2014) Arboviral bottlenecks and challenges to maintaining diversity and fitness during mosquito transmission. Viruses 6, 3991–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grubaugh ND et al. (2018) An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. bioRxiv DOI: 10.1101/383513 [DOI] [PMC free article] [PubMed]
- 50.Quick J et al. (2017) Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc 12, 1261–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer LD and Ciota AT (2015) Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol 15, 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonçalves CM et al. (2014) Distinct variation in vector competence among nine field populations of Aedes aegypti from a Brazilian dengue-endemic risk city. Parasites and Vectors 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Main BJ et al. (2018) Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS Negl. Trop. Dis 12, e0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin D et al. (2014) Transcriptomics of differential vector competence : West Nile virus infection in two populations of Culex pipiens quinquefasciatus linked to ovary development. BMC Genomics 15, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Severson DW and Behura SK (2016) Genome Investigations of Vector Competence in Aedes aegypti to Inform Novel Arbovirus Disease. Insects 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambrechts L (2011) Quantitative genetics of Aedes aegypti vector competence for dengue viruses : towards a new paradigm ? Trends Parasitol 27, 111–114 [DOI] [PubMed] [Google Scholar]
- 57.Baird NA et al. (2008) Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS One 3, e3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cianci D et al. (2013) Estimating Mosquito Population Size from Mark — Release — Recapture Data Estimating Mosquito Population Size From Mark – Release – Recapture Data. J. Med. Entomol 50, 533–542 [DOI] [PubMed] [Google Scholar]
- 59.Fouet C et al. (2018) Human Interventions: Driving Forces of Mosquito Evolution. Trends Parasitol 34, 127–139 [DOI] [PubMed] [Google Scholar]
- 60.Ritchie SA and Johnson BJ (2017) Advanves in vector control science: Rear- and-release strategies show promise… but don’t forget the basics. J. Infect. Dis 215, S103–S108 [DOI] [PubMed] [Google Scholar]
- 61.Dorigatti I et al. (2018) Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol 34, 102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie SA et al. (2018) Mission Accomplished? We Need a Guide to the “Post Release” World of Wolbachia for Aedes-borne Disease Control. Trends Parasitol 34, 217–226 [DOI] [PubMed] [Google Scholar]
- 63.Hammond A et al. (2016) A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol 34, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer JRB et al. (2017) Citizen science provides a reliable and scalable tool to track disease-carrying mosquitoes. Nat. Commun 8, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention (2013) West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. [Google Scholar]
- 66.Centers for Disease Control and Prevention (2017) Integrated mosquito management for Aedes aegypti and Aedes albopictus mosquitoes