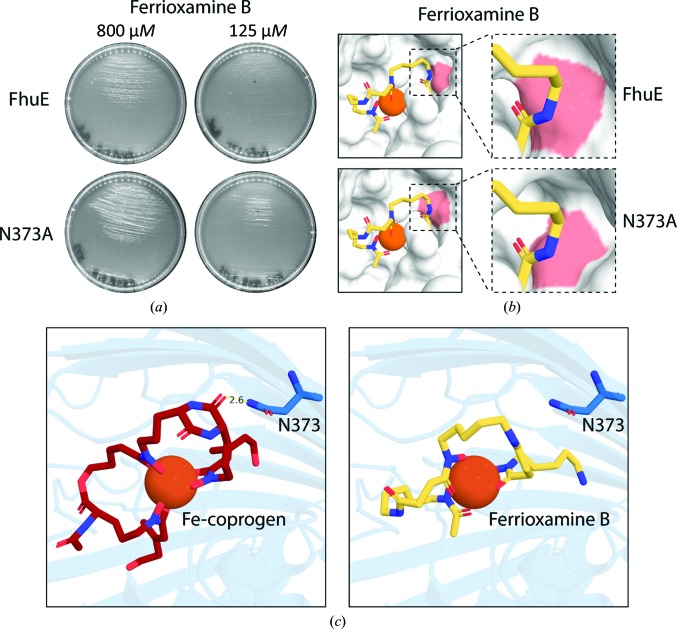
Figure 5.
Mutation of Asn373 enhances the ability of FhuE to utilize ferrioxamine B. (a) A solid agar growth assay showing the enhanced ability of the N373A mutant to support growth using ferrioxamine B as an iron source relative to the growth observed in the presence of FhuE. (b) Structural modelling of the N373A mutant with ferrioxamine B docked into the substrate-binding pocket. The removal of the asparagine side chain opens the substrate-binding channel of FhuE to better accommodate ferrioxamine B. (c) The position of Asn373 (blue sticks) relative to coprogen (left) and ferrioxamine B (right). In the crystal structure, Asn373 forms a hydrogen bond to a carbonyl group from the coprogen diketopiperazine ring. This group is not present in ferrioxamine B.