Abstract
Purpose:
Advances in cancer detection and treatment have resulted in a growing population of long-term survivors, but even years after treatment has concluded many survivors report physical symptoms that interfere with daily living. While there are studies of late effects following common cancers, less is known about these complications in rare cancers. This study focuses on the physical symptoms reported by long-term survivors enrolled in the NIH-sponsored Rare Cancer Genetics Registry.
Methods:
The Rotterdam Symptom Checklist-Modified was administered to evaluate the severity of physical symptoms commonly reported by long-term cancer survivors. Logistic regression was used to assess association between symptoms and demographic and clinical factors.
Results:
In 309 subjects with a median time of 7.6 years from a diagnosis of one or more rare cancers the median number of symptoms present per participant was 7. The most prevalent symptom reported was tiredness/lack of energy, which was present/very bothersome in 70%/25% of registrants. Women, non-whites, current smokers, and upper GI cancer survivors are particularly affected. Overall, symptom prevalence was similar across rare cancer types, time since diagnosis, and type of treatment.
Conclusions:
Rare cancer survivors continue to experience troublesome symptoms many years after diagnosis, regardless of cancer type or treatment modality.
Impact for Cancer Survivors:
There is a need for continued emphasis on smoking cessation in cancer survivors as well as enhanced monitoring of long-term complications in female, non-white, and upper GI cancer survivors.
Keywords: quality of life, Rotterdam Symptom Checklist-Modified, rare cancer, long-term survivors
BACKGROUND
As a result of improvements in detection and treatment of cancer, there has been a steady increase in the population of cancer survivors; this group accounts for over 15 million people today and is expected to increase to over 26 million by the year 2040 [1, 2]. Cancer survivors today live to an older age and are at higher risk for long-term effects of the disease and/or its treatment [3]. Cancer-related symptoms such as fatigue, insomnia, neuropathy, and pain can persist for years after the completion of treatment, yet these symptoms often remain underdiagnosed and undertreated, causing impaired function and diminishing quality of life [4]. Furthermore, it is common for survivors to experience multiple symptoms concurrently, which compounds the impact on functioning in daily life. Skerman et al reported a median of seven concurrent symptoms in a group of patients assessed one year after starting adjuvant chemotherapy [5]. Thus, there is an increasing need to identify and treat long-term morbidity in cancer survivors.
Studies have examined long-term symptom burden in common cancers [6, 7], yet over 25% of cancer diagnoses are “rare cancers” and it is challenging to conduct research in the small population of patients affected by a specific rare cancer. However, many of the late effects of a cancer diagnosis and treatment are similar across cancer types. This is because in addition to factors such as sex, age, disease stage and co-morbidities, many of the physical problems experienced by cancer survivors are due to the treatment they received. For example, radiation therapy can cause chronic pain and musculoskeletal problems and many chemotherapy regimens are associated with later reports of fatigue and peripheral neuropathy [5, 8]. Because these factors are common to all cancers, it is reasonable to combine patients with different cancer diagnoses to ensure sufficient sample sizes for analyses and generate results that reflect the impact of late symptoms in the broad population of rare cancer survivors.
Much of the existing survivorship research has focused on the treatment and early post-treatment time-frame. Clinical trials provide insight into complications patients experience but don’t often follow patients beyond five years. However, the longer-term (post-treatment) symptoms of cancer survivors, especially those with rare cancer, have received little study. This paper addresses the self-reported physical symptoms in a population of long-term survivors of rare cancers. We used a validated symptom checklist to evaluate the presence, severity, and burden of physical symptoms in participants enrolled in a national rare cancer registry and examined the demographic and clinical factors associated with presence of symptoms. Understanding the persistent and common physical symptoms in this group, as well as identifying the subgroups at greatest risk for symptoms, could provide both the patients and their health care providers an opportunity to improve the physical quality of life for the growing population of cancer survivors.
METHODS
Population:
The Rare Cancer Genetics Registry (RCGR) is a National Cancer Institute supported national research registry of individuals with rare cancers (www.rcgr.org). Since it was established in 2009, the RCGR enrolled over 800 participants at six US academic research centers. With approval from each center’s Institutional Review Board, registrants were recruited using hospital tumor registries to identify individuals diagnosed with rare hematologic, genitourinary, gastrointestinal, head/neck, gynecologic cancers or sarcoma within the previous ten years. A cancer was considered rare based on incidence of less than 20 per 100,000 in the US population.
At enrollment, registrants completed a mail or telephone survey, providing information on socio-demographic characteristics, personal and family cancer history, and cancer risk factors. Registrants were contacted annually for updates to the information they provided at baseline. Details about registrants’ diagnoses, cancer treatments and outcomes were also abstracted from electronic medical records and/or hospital tumor registry databases.
Survey:
The Rotterdam Symptom Checklist (RSCL) was developed and applied to study physical and psychological symptom burden in cancer survivors, focusing on patient-reported symptoms that are pertinent to long-term survivors [9]. A modified version of the RSCL was developed to assess a more comprehensive list of physical symptoms experienced by cancer survivors, while removing items related to the evaluation of psychological health. This RSCL-M (Rotterdam Symptom Checklist-Modified) includes symptoms associated with several common cancer sites, making it relevant in studies of patients with a wide range of cancers and varying treatment. The modified scale was found to be reliable and valid for use with cancer patients in the US [10].The RSCL-M asks respondents to indicate the extent to which they have been bothered by each of 28 physical symptoms during the past week by selecting “not at all” (coded as 1), “a little” (2), “quite a bit” (3), and “very much” (4). In 2017 the RCGR contacted current registrants and asked them to complete the RSCL-M.
Statistical Methods:
Symptom responses were dichotomized as absent (1) versus present (2–4). We also used a second dichotomization for symptom response. Noting that the RSCL-M assesses how much respondents are bothered by each symptom, we dichotomized the responses as very bothersome (3–4) versus not very bothersome (1–2). Finally, we computed the number of symptoms present, the number of very bothersome symptoms, and the total symptom score over all 28 symptoms for each participant. Univariate and multivariate logistic regression models were used to assess associations between each patient-reported dichotomized symptom (present versus absent) and socio-demographic and cancer-related characteristics. The symptoms of tiredness and lack of energy were combined to create a single symptom. Age was dichotomized by age 65, as was race (white), current tobacco and current alcohol use. Separate indicator (yes/no) variables were used to group cancer diagnosis by type—sarcoma, hematologic, cancers affecting organs of the upper gastrointestinal tract, urinary system, and head and neck. Registrants with multiple diagnoses were included in multiple cancer groups in the regression models. Time since diagnosis was dichotomized by 5 years, using most recent diagnosis for those with multiple primaries, and treatment variables were included, indicating whether any of the registrant’s cancers were treated with surgery, chemotherapy or radiation.
For each symptom, a single logistic regression analysis, combined across cancer types, was performed. Wald tests were used to determine whether the estimated odds ratios (ORs) were significantly different from one (no association). Backwards selection was used to derive the final multivariate model for each symptom. To ensure adequate power for the multivariate analyses, only symptoms present in more than 100 participants (33%) were evaluated for association with participant characteristics. Participants with missing or unknown values for a characteristic were excluded from analyses of the given characteristic. In addition to assessing symptoms individually, we also evaluated which characteristics are associated with high (more than median number of symptoms) versus low symptom burden in the same manner as described above. The datasets analyzed for this study are available from the corresponding author on request.
RESULTS
Registrants:
As of 2017, 515 of the over 800 registrants of the Rare Cancer Genetics Registry (RCGR) were still actively participating, and were mailed the RSCL-M. Sixty percent (309) completed the checklist. Characteristics of the participants in this study are presented in Table 1. Details of the types of diagnoses are provided in Online Resource 1. The 309 participants had a total of 350 cancer diagnoses, including a broad array of rare cancers as well as some additional common cancers among the 31 participants (10%) with multiple diagnoses. The median age at most recent cancer diagnosis was 57 years (range: 21–87) and the median time since most recent diagnosis was 7.6 years (range: 1.4–27.5). The majority of participants were male (54%), reported white race (89%), were not current smokers (95%), and drank some alcohol (66%). Most participants had undergone surgery to treat their cancer(s) (88%), and about half were treated with chemotherapy (57%) and/or radiotherapy (58%). A small number of participants (13%) were still undergoing treatment for their cancers. Regarding the reasons for non-response, the registrants who were mailed the checklist but did not respond were significantly more likely to have multiple cancers, be more than five years since their most recent diagnosis, and not to have undergone surgery for their cancer (Fisher’s exact test for all comparisons). The response rate was not related to current age, gender, race, Hispanic ethnicity, smoking history, alcohol consumption, age at most recent cancer diagnosis, or treatment with chemotherapy or radiation.
Table 1.
Participant Characteristics (N = 309)
| Characteristic | N (%) |
|---|---|
| Age at RSCL-M completion (years) | |
| Female gender | 142 (46) |
| Race1 | |
| Hispanic ethnicity1 | 6 (2%) |
| Tobacco use1 | |
| Alcohol consumption (drinks per week)1 | |
| Rare cancer type | |
| Number of cancer diagnoses | |
| Age at most recent diagnosis (years)1 | |
| Currently receiving treatment | 40 (13) |
| ≥ 5 years since most recent diagnosis | 232 (75) |
| Any cancer treated with surgery | 272 (88) |
| Any cancer treated with chemotherapy | 175 (57) |
| Any cancer treated with radiotherapy | 140 (45) |
Characteristic (number with unknown values): race (6), Hispanic (4), tobacco (2), alcohol (1), age at diagnosis (7)
Includes esophagus (n=47), biliary tract (21), stomach (20), pancreas (8)
Includes ovary/fallopian tube/peritoneum (7), uterus/endometrium (3)
Prevalence of Symptoms:
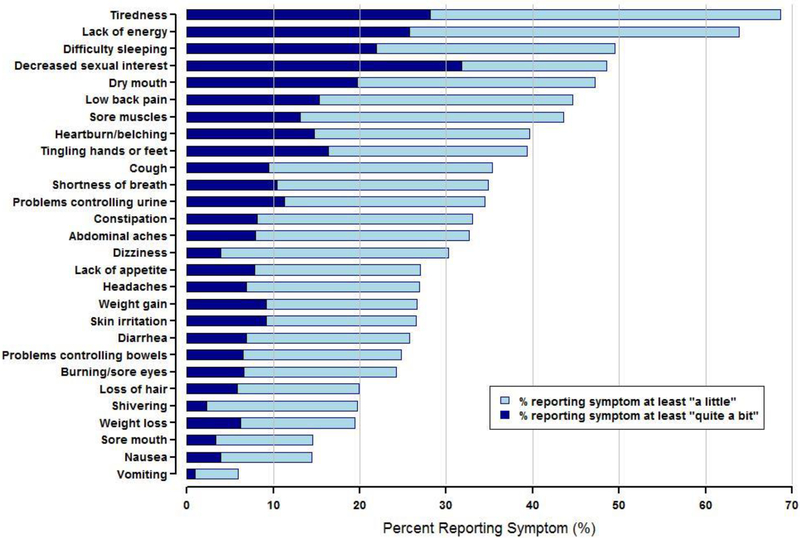
Figure 1 shows the proportion of participants reporting each symptom using two different dichotomizations: those reporting being bothered by the symptom at least “a little” (2–4; i.e. present) and those reporting the symptoms at least “quite a bit” (3–4; i.e. very bothersome). In the examination of whether registrants reported the symptom at least “a little,” tiredness and lack of energy were the most commonly-reported symptoms with each present in more than 60% of participants. Difficulty sleeping, decreased sexual interest, dry mouth, low back pain, and sore muscles were present in 40–50% of participants, while nausea, sore mouth, and vomiting were uncommon and present in less than 20% of participants. The proportions of registrants who reported being bothered by a given symptom “quite a bit” or “very much” (i.e. symptom was very bothersome) are much lower than the proportions who report the symptom at least “a little.” and only exceeded 20% for decreased sexual interest (very bothersome in 32% of participants), tiredness (28%), lack of energy (26%), and difficulty sleeping (22%) (Figure 1).
Figure 1.
Prevalence of Symptoms in All Participants (N=309). Symptoms are ordered from most to least prevalent.
Table 2 presents the proportions of participants with each cancer type who reported symptoms (present at least “a little”). Tiredness/lack of energy was present in more than 50% of participants in every cancer type except testis & prostate. Difficulty sleeping was very commonly present in several cancers, although markedly more frequent in breast (65%) and gynecologic (69%) cancers. Decreased sexual interest was also most commonly present in cancers affecting women and was reported in 72% of participants with breast cancer and 67% of participants with gynecologic cancers. Dry mouth was very often present among head/neck cancers (78%), while heartburn/belching was most frequently present in participants with cancers of the stomach (60%) and biliary tract, liver and pancreas (59%). Looking across all symptoms, the median number of symptoms present in a participant was 7 (range: 0–24), while the median number of very bothersome symptoms per participant was 2 (range: 0–16). The mean total symptom score was 39.0 (scale: 28–112, with a score of 28 corresponding to no symptoms). Mean symptom scores ranged from slightly over one for uncommon symptoms to approximately two for the three most prevalent symptoms and were very similar to those observed in the RSCL-M validation study population (Online Resource 2). Participants with cancers of the biliary tract, liver and pancreas had the highest total symptom score (41.8 on a scale of 28–112), while participants with kidney/bladder cancers and testis/prostate cancers had the lowest total scores (36.2 and 32.1, respectively) (Table 2). Most of the cancer-specific mean total scores were within 2 points of the overall mean of 39.0. Participants with cancers of the biliary tract, liver and pancreas reported the highest number of symptoms present (mean=11), while those with kidney/bladder cancers and testis/prostate cancers reported the fewest symptoms present (means=6.5 and 4.1, respectively).
Table 2.
Total Symptom Score and Prevalence of Symptoms by Cancer Type. Cancer types are sorted from highest to lowest mean total symptom score. Symptoms present in > 33% of participants are shown; symptoms present in > 50% of participants are highlighted in gray. Registrants with multiple cancers appear in multiple rows.
| Cancer type (# participants) | Total symptom score Mean (SE) | Percent with symptom | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tiredness/ lack of energy |
Difficulty sleeping |
Decreased sexual interest |
Dry mouth |
Low back pain |
Sore muscles |
Heartburn/ belching |
Tingling | Cough | Shortness of breath |
Problems controlling urine |
||
| Biliary tract, liver & pancreas (30) | 41.8 (1.9) | 79% | 59% | 62% | 45% | 52% | 55% | 59% | 41% | 28% | 41% | 50% |
| Esophagus (47) | 40.6 (1.7) | 68% | 52% | 41% | 45% | 45% | 43% | 50% | 45% | 40% | 53% | 30% |
| Thymus (l0) | 40.5 (3.1) | 70% | 50% | 50% | 40% | 60% | 60% | 50% | 60% | 40% | 50% | 20% |
| Hematologic (24) | 40.1 (2.8) | 78% | 52% | 59% | 43% | 52% | 43% | 27% | 58% | 43% | 43% | 39% |
| Breast (20) | 40.1 (1.8) | 83% | 65% | 72% | 56% | 39% | 61% | 39% | 44% | 39% | 33% | 33% |
| Gynecologic (13) | 39.8 (3.1) | 62% | 69% | 67% | 46% | 46% | 58% | 31% | 50% | 38% | 15% | 0% |
| Stomach (20) | 39.7 (2.0) | 80% | 60% | 37% | 45% | 50% | 53% | 60% | 40% | 20% | 35% | 35% |
| Small bowel & colon (8) | 39.5 (4.5) | 57% | 29% | 29% | 29% | 29% | 29% | 29% | 29% | 14% | 29% | 14% |
| Sarcoma (54) | 39.2 (1.4) | 72% | 43% | 62% | 42% | 53% | 47% | 38% | 42% | 34% | 32% | 51% |
| Head/neck | 38.1 (1.3) | 65% | 54% | 43% | 78% | 33% | 37% | 29% | 23% | 45% | 20% | 25% |
| Kidney & bladder (48) | 36.2 (1.4) | 65% | 42% | 40% | 38% | 40% | 30% | 33% | 31% | 30% | 30% | 33% |
| Testis & prostate (15) | 32.1 (1.8) | 40% | 13% | 13% | 27% | 27% | 27% | 20% | 27% | 7% | 27% | 33% |
| All participants (309) | 39.0 (10.2) | 70% | 50% | 48% | 47% | 45% | 44% | 40% | 39% | 35% | 35% | 34% |
Total mean symptom score was calculated by summing response values across all 28 symptoms; possible score range from 28–112.
Factors Associated with Presence of Symptoms:
The odds ratios (ORs) from logistic regression models for the association of presence of symptoms with demographic and cancer-related factors are given in Table 3. Demographic characteristics including age, gender, race, current smoking, and alcohol consumption were significantly associated with presence of several symptoms. Female participants were significantly more likely to report lack of energy/tiredness (OR=1.69), difficulty sleeping (OR=1.97), and decreased sexual interest (OR=2.09). Non-white participants reported more sore muscles than white participants (OR=2.77). Current smokers were markedly more likely to report presence of many of the symptoms we evaluated, with the estimated odds of symptoms in current smokers ranging from 2.8 to 8.6 times greater than the odds of symptoms in non-smokers. In addition, current smokers, female, or non-white subjects were more likely to report high symptom burden (i.e. > 7 symptoms present in an individual). Participants who drink at least one alcoholic beverage per week were significantly less likely to report symptoms including low back pain (OR=0.56), tingling hands or feet (OR=0.52), and problems controlling urine (OR=0.57) compared to participants who reported no alcohol consumption. In the evaluation of the association between symptoms and cancer treatments, we found that participants who received chemotherapy were at increased risk for sore muscles (1.60) and tingling (OR=1.98), while neither treatment with surgery nor radiation were associated with any of the symptoms we examined.
Table 3.
Factors Associated with Presence of Symptoms. Univariate logistic regression odds ratios for symptom presence in participants with a given characteristic versus those without the characteristic are shown. The level of significance (p-value) from univariate Wald tests is indicated by the number of asterisks: (* = 0.05 ≤ p < 0.1, ** = 0.01 ≤ p < 0.05, and *** = p < 0.01). Associations that remained significant (p<0.05) in a multivariate logistic regression model are shown in bold and underlined.
| Characteristic | Odds ratio for association of symptom with characteristic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tiredness/ lack of energy |
Difficulty sleeping |
Decreased sexual interest |
Dry mouth |
Low back pain |
Sore muscles |
Heartburn/ belching |
Tingling hands/feet |
Shortness of breath |
Cough | Problems controlling urine |
More than 7 symptoms |
|
| Age at survey ≥ 65 | 1.20 | 0.98 | 1.00 | 0.92 | 1.32 | 0.65* | 1.12 | 1.21 | 1.80** | 1.03 | 2.32*** | 1.21 |
| Female gender | 1.69** | 1.97*** | 2.09*** | 1.11 | 1.06 | 1.45 | 0.78 | 1.43 | 0.79 | 1.07 | 1.13 | 1.75** |
| Non-white race | 1.70 | 1.81 | 1.32 | 1.10 | 2.05* | 2.77*** | 1.13 | 1.68 | 1.46 | 0.94 | 1.86 | 2.02* |
| Current smoker | 2.69 | 2.70 | 2.80* | 4.36** | 8.08*** | 8.57*** | 4.07** | 1.83 | 7.56*** | 3.58** | 4.64** | 6.64** |
| Drinks alcohol | 0.79 | 0.79 | 0.93 | 0.82 | 0.56** | 0.60** | 0.64* | 0.52*** | 0.79 | 0.76 | 0.57** | 0.57** |
| Multiple cancers | 0.71 | 0.81 | 1.15 | 0.98 | 1.10 | 0.99 | 0.78 | 0.88 | 0.93 | 0.43* | 1.00 | 0.82 |
| Age at diagnosis1 ≥ 65 | 1.30 | 0.97 | 1.16 | 1.24 | 1.31 | 0.81 | 1.40 | 1.30 | 1.86** | 1.60* | 2 09*** | 1.35 |
| ≥ 5 years since diagnosis1 | 1.08 | 0.85 | 0.80 | 1.10 | 1.47 | 1.12 | 1.06 | 1.02 | 1.47 | 1.01 | 1.29 | 1.33 |
| Underwent surgery | 0.89 | 1.43 | 1.00 | 0.60 | 1.15 | 1.09 | 1.36 | 0.54* | 0.63 | 0.84 | 0.87 | 0.92 |
| Received chemotherapy | 1.07 | 1.52* | 1.21 | 1.61** | 1.13 | 1.60** | 1.31 | 1.98*** | 0.96 | 1.39 | 0.68 | 1.52* |
| Received radiotherapy | 0.97 | 1.15 | 1.12 | 1.48* | 0.79 | 1.06 | 1.12 | 0.89 | 0.82 | 1.25 | 0.86 | 0.89 |
| Sarcoma | 1.10 | 0.74 | 1.90** | 0.79 | 1.49 | 1.19 | 0.94 | 1.12 | 0.86 | 0.93 | 2.32*** | 0.89 |
| Upper GI | 1.32 | 1.44 | 0.90 | 0.87 | 1.21 | 1.33 | 2.47*** | 1.23 | 1.99*** | 0.82 | 1.20 | 1.62* |
| Urinary | 0.77 | 0.70 | 0.65 | 0.65 | 0.78 | 0.51* | 0.70 | 0.65 | 0.78 | 0.77 | 0.94 | 0.78 |
| Head/neck | 0.77 | 1.25 | 0.79 | 4.89*** | 0.55* | 0.71 | 0.56* | 0.40** | 0.43** | 1.62 | 0.59 | 0.80 |
| Hematologic | 1.59 | 1.12 | 1.59 | 0.85 | 1.39 | 0.99 | 0.55 | 2.31* | 1.48 | 1.45 | 1.25 | 1.02 |
Defined as age at/time since most recent diagnosis
In general, cancer type was not related to risk for most symptoms, although a few associations were significant. A diagnosis of head and neck cancer was associated with a greater than four-fold increase in odds of dry mouth compared to other diagnoses (OR=4.89). Similarly, participants with cancer affecting the upper gastrointestinal (GI) tract were significantly more likely to report heartburn/belching (OR=2.73) and shortness of breath (OR=1.99) than those with other cancers. Upper GI cancers were also more likely to experience high symptom burden compared to other cancer types.
DISCUSSION
The study of late effects in rare cancers is difficult due to the small number of affected patients and the lack of long-term follow-up in these populations. The Rare Cancer Genetics Registry provided a unique opportunity to assess the prevalence of symptoms in a large group of rare cancer survivors, most of whom are many years from the diagnosis and conclusion of treatment. Combining information from registrants with several types of cancer allows us to compare the occurrence of symptoms both within and between cancer sites, as well as providing greater statistical power for the analyses.
We found that most participants experience tiredness and lack of energy. At a median of over 7 years since their most recent cancer diagnosis, nearly 70% of participants reported presence of these fatigue-related symptoms and over 25% indicated these symptoms were very bothersome. Difficulty sleeping was the next most commonly-reported symptom, with 50% of participants endorsing presence of insomnia. This is consistent with other studies that have found fatigue and insomnia to be among the most common symptoms experienced by cancer survivors [2, 4]. Decreased sexual interest was also quite common and had the highest rate (32%) of participants who were very bothered by the symptom. Similar results have been observed in prior studies that have reported that sexual problems can persist for years after cancer diagnosis and treatment [11, 12]. The least prevalent symptoms were those that are typically present only during active treatment with chemotherapy and/or radiation, including nausea, vomiting, weight loss, shivering, and hair loss. This is not unexpected since most RCGR subjects were remote from diagnosis and thus unlikely to be receiving treatments. Although many symptoms were present in a substantial proportion of participants, most symptoms were reported as being very bothersome in only a small fraction of participants. Furthermore, while the median number of symptoms present in a participant was 7, the median number of symptoms that were very bothersome was 2, suggesting that rare cancer survivors typically experience several symptoms at fairly low levels of severity (“a little”) with a few that are very bothersome. These results are similar to what has been reported for common cancers and mirror the findings in the RSCL-M validation population, despite the considerably longer time since diagnosis in the RCGR population [10].
Our results did not suggest substantial variation in the prevalence of symptoms due to cancer type. The cancer-specific mean total symptom scores were mostly clustered within a few points of the overall mean. The associations we observed were between symptoms linked to a specific site in the body that was affected by the cancer, such as registrants with head and neck cancers, who were almost five times more likely to report dry mouth. Despite recent improvements in radiotherapy techniques, late radiation-induced dry mouth remains one of the main morbidities affecting head and neck cancer survivors [13, 14]. Similarly, those with upper GI cancers were significantly more likely to report heartburn/belching, and surgical resection (a common treatment in GI cancers) is often linked to reflux symptoms such as heartburn and belching in patients with esophagogastric cancers [15, 16]. In our multivariate analysis that combined all patients regardless of cancer site, we did not detect any symptoms that were significantly associated with either surgery or radiation. However, participants treated in the past with chemotherapy experienced more tingling in the hands and feet than those who did not undergo chemotherapy, which is consistent with evidence that some chemotherapeutic agents are associated with long-term peripheral neuropathy [17].
Women reported higher rates of lack of energy/tiredness, difficulty sleeping, and decreased sexual interest in the multivariate analysis. These associations have been found in previous studies of cancer survivors that showed women were more likely to report cancer-related fatigue [18, 19] and sexual problems [20] in various cancer diagnoses. Non-whites were at elevated risk for sore muscles and high symptom burden, which is consistent with existing research showing disparities in pain control between white and non-white survivors and reports showing that cancer survivors in minority groups are less likely to seek medical care for management of physical symptoms [21]. Although our study only included a small number of current smokers, we found strong associations between current smoking and several symptoms, and smokers were much more likely to report a high number of symptoms. Other studies have reported poorer health-related quality of life (QOL) for cancer survivors who smoke [22]. In particular, odds ratio estimates for low back pain and sore muscles comparing current smokers to others both exceeded eight in the multivariate analysis, findings which are supported by a previous study that showed continued smoking despite a cancer diagnosis was associated with greater pain severity and interference from pain [23]. Unexpectedly, alcohol consumption was associated with decreased risk of low back pain, tingling hands/feet, and problems controlling urine. Guidelines for cancer survivors typically include limiting alcohol consumption due to the link between drinking and risk of recurrence and new primaries [23], but the relationship between alcohol consumption and health-related QOL in cancer survivors is unclear, with at least one study finding no association between alcohol use and several QOL outcomes [24].
Our study had several limitations. The cross-sectional study design did not permit us to evaluate temporal patterns in symptoms following a rare cancer diagnosis. The response rate was 60%, with nonresponders more likely to be more than five years from diagnosis. We cannot rule out survivorship bias in our results, as participants had to live long enough past their diagnosis to be enrolled in the registry and subsequently complete the symptom survey. Further, our Rare Cancer Genetics Registry was a convenience sample and not demographically representative of the population from which it was drawn. Also, while it was necessary to combine registrants with many different types of rare cancer to ensure adequate power for the multivariate analysis, this aggregation is potentially problematic due to fundamental differences in presentation, disease course and treatment across diagnoses. We accounted for these differences by including cancer group (by anatomy) and treatment types in the models, and note that given that most participants are several years from diagnosis it is not surprising that these factors were not among the major predictors of symptoms. Finally, we did not assess participants’ physical functioning so it is unclear what impact the presence, severity, or number of symptoms has on their ability to function in daily life. We note lastly that in addition to the physical symptoms we assessed, long-term survivors remain at risk for late secondary cancers as well as psychological problems, but these symptoms are also outside the realm of this research.
In conclusion, we found that most rare cancer survivors continue to experience troublesome symptoms many years after diagnosis and over half remain very bothered by multiple symptoms. These findings highlight a need for proactive strategies to prevent and manage symptoms during both the active treatment and survivorship phases of care. Such strategies could include utilizing physical rehabilitation and psychosocial counseling interventions that can mitigate or even prevent the development and progression of many of the most commonly-reported symptoms in our study. With a few exceptions, symptom prevalence was similar across rare cancer types. Current smokers, non-whites, female, and GI cancer survivors were at increased risk for symptoms, suggesting the need for continued emphasis on smoking cessation in survivor populations and enhanced clinical evaluation and development of interventions targeted toward minority, female and GI cancer survivors.
Supplementary Material
Acknowledgements:
The authors would like to gratefully acknowledge the Rare Cancer Genetics Registry participants and project managers, as well as the Principal Investigators of the Rare Cancer Genetics Registry: Susan Domchek (University of Pennsylvania), Claudine Isaacs (Georgetown University), Jan Lowery & Betsy Risendal (University of Colorado Denver), Patricia Moorman (Duke University), Kala Visvanathan (Johns Hopkins University)
Funding: This study was funded by the U.S. National Institutes of Health (grants RC1 CA144706 and R01 CA160233).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283(22):2975–8. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017;18(1):e11–e8. doi: 10.1016/S1470-2045(16)30573-3. [DOI] [PubMed] [Google Scholar]
- 4.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–96. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 5.Skerman HK, Yates PM, Battitutta D. Cancer-related symptom clusters for symptom management in outpatients after commencing adjuvant chemotherapy, at 6 months, and 12 months. Support Care Cancer. 2012;20(1):95–105. doi: 10.1007/s00520-010-1070-z. [DOI] [PubMed] [Google Scholar]
- 6.Tian Y, Schofield PE, Gough K, Mann GB. Profile and predictors of long-term morbidity in breast cancer survivors. Ann Surg Oncol. 2013;20(11):3453–60. doi: 10.1245/s10434-013-3004-8. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Cheville AL, Wampfler JA et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol. 2012;7(1):64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs. 2001;17(4):241–8. [DOI] [PubMed] [Google Scholar]
- 9.de Haes JC, van Knippenberg FC, Neijt JP. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer. 1990;62(6):1034- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein KD, Denniston M, Baker F, Dent M, Hann DM, Bushhouse S et al. Validation of a modified Rotterdam Symptom Checklist for use with cancer patients in the United States. J Pain Symptom Manage. 2003;26(5):975–89. [DOI] [PubMed] [Google Scholar]
- 11.Meyerowitz BE, Desmond KA, Rowland JH, Wyatt GE, Ganz PA. Sexuality following breast cancer. J Sex Marital Ther. 1999;25(3):237–50. doi: 10.1080/00926239908403998. [DOI] [PubMed] [Google Scholar]
- 12.Syrjala KL, Roth-Roemer SL, Abrams JR, Scanlan JM, Chapko MK, Visser S et al. Prevalence and predictors of sexual dysfunction in long-term survivors of marrow transplantation. J Clin Oncol. 1998;16(9):3148–57. doi: 10.1200/JCO.1998.16.9.3148. [DOI] [PubMed] [Google Scholar]
- 13.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 14.Head MDA, Neck Cancer Symptom Working G, Kamal M, Rosenthal DI, Volpe S, Goepfert RP et al. Patient reported dry mouth: Instrument comparison and model performance for correlation with quality of life in head and neck cancer survivors. Radiother Oncol. 2018;126(1):75–80. doi: 10.1016/j.radonc.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allum WH, Bonavina L, Cassivi SD, Cuesta MA, Dong ZM, Felix VN et al. Surgical treatments for esophageal cancers. Ann N Y Acad Sci. 2014;1325:242–68. doi: 10.1111/nyas.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouras G, Markar SR, Burns EM, Huddy JR, Bottle A, Athanasiou T et al. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur J Surg Oncol. 2017;43(2):454–60. doi: 10.1016/j.ejso.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. 2017;166(2):519–26. doi: 10.1007/s10549-017-4437-8. [DOI] [PubMed] [Google Scholar]
- 18.Hwang IC, Yun YH, Kim YW, Ryu KW, Kim YA, Kim S et al. Factors related to clinically relevant fatigue in disease-free stomach cancer survivors and expectation-outcome consistency. Support Care Cancer. 2014;22(6):1453–60. doi: 10.1007/s00520-013-2110-2. [DOI] [PubMed] [Google Scholar]
- 19.Thong MSY, Mols F, van de Poll-Franse LV, Sprangers MAG, van der Rijt CCD, Barsevick AM et al. Identifying the subtypes of cancer-related fatigue: results from the population-based PROFILES registry. J Cancer Surviv. 2018;12(1):38–46. doi: 10.1007/s11764-017-0641-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim IR, Kim SH, Ok ON, Kim SH, Lee S, Choi E et al. Sexual problems in male vs. female non-Hodgkin lymphoma survivors: prevalence, correlates, and associations with health-related quality of life. Ann Hematol. 2017;96(5):739–47. doi: 10.1007/s00277-017-2940-y. [DOI] [PubMed] [Google Scholar]
- 21.Blinder VS, Griggs JJ. Health disparities and the cancer survivor. Semin Oncol. 2013;40(6):796–803. doi: 10.1053/j.seminoncol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard CM, Courneya KS, Stein K, American Cancer Society’s SCS, II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 23.Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–5. doi: 10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.