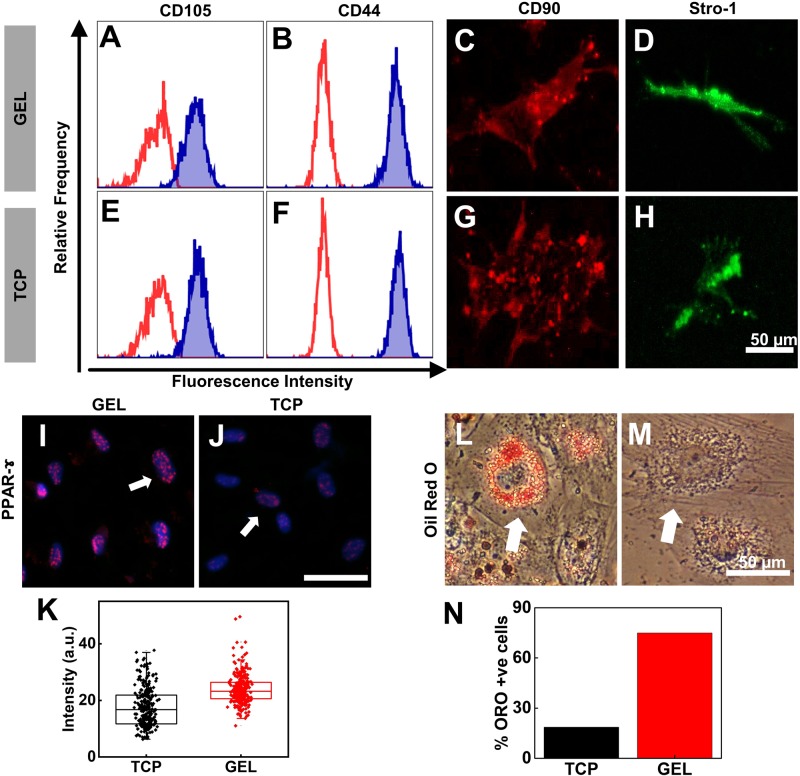
Fig. 4.
Long-term culture on gel did not alter surface marker expression but helps stem cells maintain differentiation potential. Flow cytometry analysis of the expression of surface pluripotency markers of UC-hMSCs at the LP (P22) from the gel (A–D) and TCP (E–H) was determined. The expression of surface markers, CD105, CD44, CD90, and Stro-1 was not altered by the long-term culture of UChMSCs on the gel. The red curve in A, B, E and F is auto-fluorescence of the cells and the blue filled histogram is the fluorescence signal of the stained marker. (I–N) Adipogenic differentiation: LP hMSCs (P14) from gel and TCP were cultured in adipogenic induction media. (I–K) The early adipogenic marker PPAR-γ expression was checked after 7 days of adipo-induction using immunostaining of UC-hMSCs from gel (I) and TCP (J) (magenta puncta in the blue nucleus, shown by white arrow). The UC-hMSCs serially passaged on gel showed higher expression of PPAR-γ compared to the cells cultured on TCP, as quantified in K (N=2, n=273, ***P<0.001). (L–N) After 14 days of adipogenic induction, the lipid droplets accumulation was significantly higher in cells from the gel (L) than from TCP (M). Lipid droplets were identified by staining with Oil Red O. The percentage of Oil Red O-positive cells was higher on the gel substrate compared to TCP, as quantified in N (N=2, n=100). White arrow shows cells with and without oil droplet accumulation. Scale bars: 50 µm.