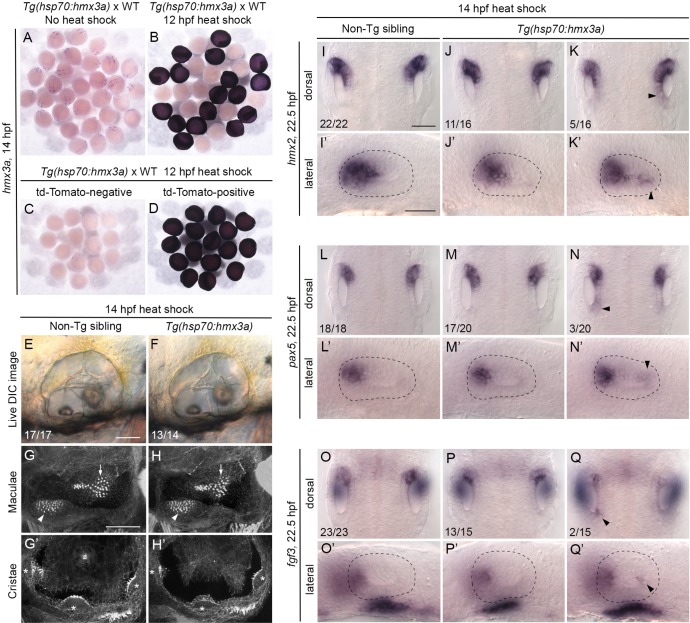
Fig 6. Mis-expression of hmx3a is not sufficient to generate an anterior ear duplication.
(A–D) Control experiments to check for successful expression of the hmx3a transgene after heat shock at 12 hpf. Embryos were fixed and stained by in situ hybridisation two hours later, at 14 hpf. (A,B) A mixed batch of embryos from a cross between a fish hemizygous for the transgene and a wild type (WT). All embryos (31/31) showed the normal pattern of expression of hmx3a in the absence of heat shock (A). After a 60-minute heat shock at 12 hpf, ~50% of the batch (17/30) showed strong, systemic expression of the transgene at 14 hpf, as expected (B). All embryos shown in B were stained in the same tube. (C,D) Embryos heat-shocked for 60 minutes at 12 hpf were sorted on the basis of tdTomato expression before fixing. All tdTomato-negative embryos (14/14) were also negative for expression of the hmx3a transgene (C); all tdTomato-positive embryos (16/16) were also positive for hmx3a transgene expression (D). (E,F) Live DIC images of ears of non-transgenic (E) and transgenic (F) sibling embryos at 3 dpf (72 hpf), after a 30-minute heat shock at 14 hpf. (G–H’) Confocal images of FITC-phalloidin-stained ears at 3 dpf (72 hpf). Position and size of the two maculae (G,H, arrowheads and arrows) and three cristae (G’,H’, asterisks) were normal in both non-transgenic and transgenic sibling embryos after heat shock. (I–Q’) In situ hybridisation for otic marker genes in non-transgenic and Tg(hsp70:hmx3a) sibling embryos after a 30-minute heat shock at 14 hpf. Dorsal views of both otic vesicles (I–Q) and lateral views of a single otic vesicle (I’–Q’) are shown. Note weak ectopic expression of hmx2 and pax5, and posterior otic expression of fgf3, in the otic vesicles of a minority of transgenic embryos (right hand column; arrowheads). Numbers of embryos showing the phenotypes are shown for each panel. WT, wild type (AB strain). Scale bar in E, 50 μm (applies to E–F); scale bar in G, 50 μm (applies to G–H’); scale bar in I, 50 μm (applies to I–Q); scale bar in I’, 50 μm (applies to I’–Q’).