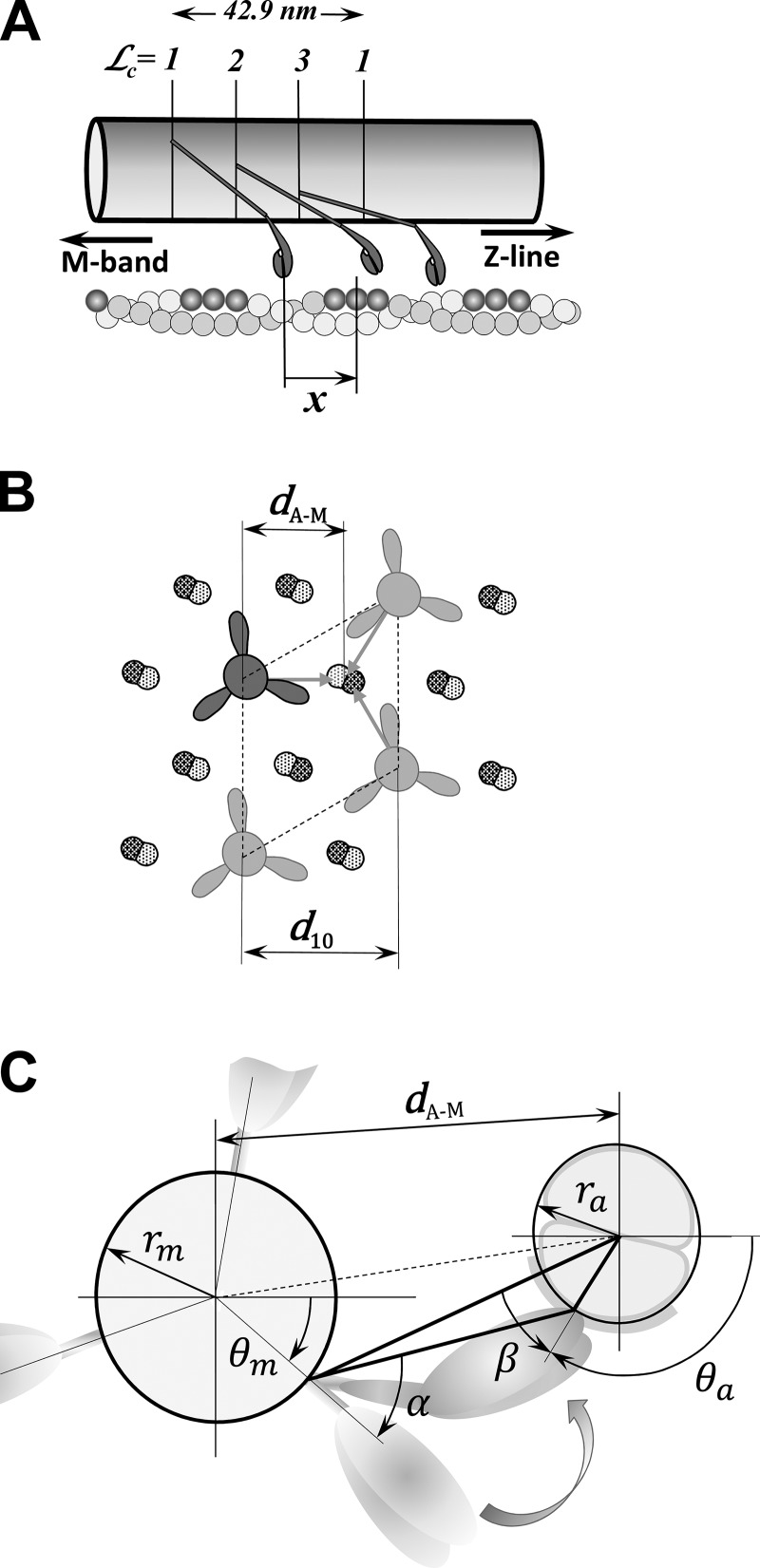
Figure 3.
The relative distance between a myosin head and the adjacent binding site on actin in the 3-D sarcomere lattice. (A) Myosin molecules form a triple helix along the myosin filament where the myosin heads are arranged in layers and at each layer form a “crown” with three myosin molecules. The crowns = 1, 2, and 3 are axially separated by 14.3 nm and rotated by 40°, forming different angular arrangements with actin filaments, but only those that might interact with the actin filament are shown. On the other side, the actin monomers are helically arranged in a double-strand helical structure, and favorable myosin-binding sites on actin filament associated with nearby myosin heads are shown in dark gray. In the axial direction, each myosin pair of heads, denoted as a cross-bridge and multiple binding sites on surrounding actin filaments, forms a large number of arrangements defined by the change in relative axial distances, x, between the unstrained position of the cross-bridge and the nearest actin-binding site. (B) The hexagonal sarcomere lattice with 2:1 actin-to-myosin-filament ratio shows in the azimuthal plane that up to three myosins can attach to each actin filament. The lattice size is defined by the distance of the 1,0 reflection from the center of the x-ray pattern, denoted as d1,0, and calculated distance between axes of myosin and actin filaments, dA–M. (C) The spatial arrangement of a crown interacting with six surrounding actin filaments is shown. The heads in crowns = 1, 2, and 3 have different azimuthal spatial arrangement relative to binding sites on the actin filaments displayed by separation by distance dA–M and azimuthal angles α and β. The figures are adapted from Mijailovich et al. (2016).