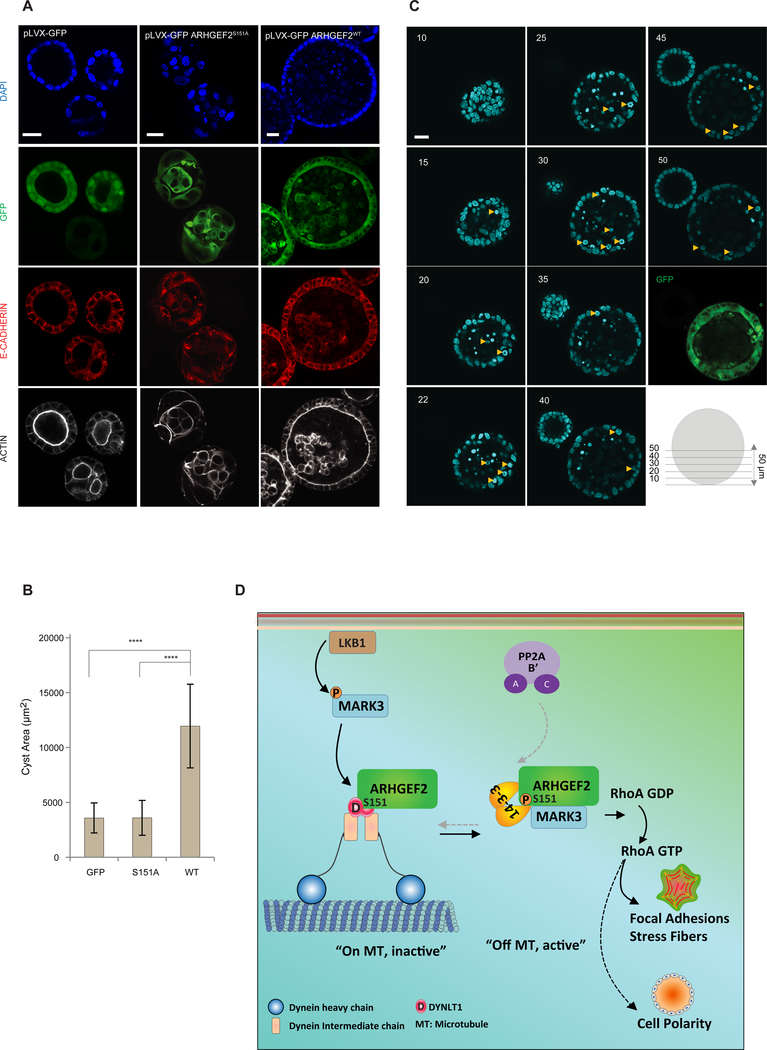
Figure 8. Phosphorylation of ARHGEF2 Ser151 is required for normal cell polarity.
(A-C) 3D culture of MDCKII cells stably expressing inducible pLVX-GFP, pLVX-GFP ARHGEF2WTand pLVX-GFP ARHGEF2S151A. In (A), GFP fluorescence was visualized and cysts were stained for E-CADHERIN, ACTIN and DAPI. In (B), average size of the cysts observed in pLVX-GFP, pLVX-GFP ARHGEF2WTand pLVX-GFP ARHGEF2S151A (n=24; 21; 24 respectively). Data are are means ± SD of three independent experiments. Statistical significance was determined by a one way ANOVA test with a Bonferroni post-test correction for multiple comparisons. ****P≤0.0001. Scale bar, 20 μm. In (C) 1 μm Z-stacks of pLVX-GFP ARHGEF2WT-cysts. Abnormal mitotic events are indicated (yellow arrows). Note that the cyst on the left has lost expression of pLVX-GFP ARHGEF2wt. Numbers represent the Z-stack step. Images are representative of four independent experiments. Scale bar, 20 μm.
(D) Schematic representation summarizing the effects of MARK3 and PP2A in the regulation of ARHGEF2 phosphorylation and its effects on RHOA activation. LKB1 activates MARK3 that in turn phosphorylates ARHGEF2 on Ser151. This creates a 14-3-3 binding site that disrupts ARHGEF2 interaction with DYNLT1 and releases it from microtubules to activate RHOA and formation of stress fibers and focal adhesions. MARK3 phosphorylation of Ser151 is required for epithelial cell polarity in three-dimensional growth. PP2A dephosphorylates Ser151 through interactions with the B’ subunits.