SUMMARY
DEET (N, N-diethyl-meta-toluamide) is the most effective and widely used insect repellent, but its mechanism of action is both complex and controversial [1]. DEET acts on insect smell [2–6] and taste [7–11], and its olfactory mode of action requires the odorant co-receptor orco [2, 3, 6]. We previously observed that orco mutant female Aedes aegypti mosquitoes are strongly attracted to humans even in the presence of DEET, but are rapidly repelled after contacting DEET-treated skin [12]. DEET inhibits food ingestion by Drosophila melanogaster flies and this repellency is mediated by bitter taste neurons in the proboscis [9]. Similar neurons were identified in mosquito proboscis, leading to the hypothesis that DEET repels on contact by activating an aversive bitter taste pathway [10]. To understand the basis of DEET contact chemorepellency, we carried out behavioral experiments and discovered that DEET acts by three distinct mechanisms: smell, ingestion, and contact. Like bitter tastants, DEET is a feeding deterrent when ingested. But its bitterness per se does not fully explain DEET contact chemorepellency. Mosquitoes blood-fed on human arms treated with high concentrations of bitters, but rapidly avoided DEET-treated skin and did not blood-feed. Insects detect tastants both through their proboscis and legs. We show that DEET contact chemorepellency is mediated exclusively by the tarsal segments of the legs, and not the proboscis. This work establishes mosquito legs as the behaviorally relevant contact sensors of DEET. These results will inform the search for molecular mechanisms mediating DEET contact chemorepellency and novel contact-based insect repellents.
Keywords: DEET, insect repellent, mosquito, Aedes aegypti, bitter, ingestion, contact chemorepellency, olfaction
Graphical Abstract
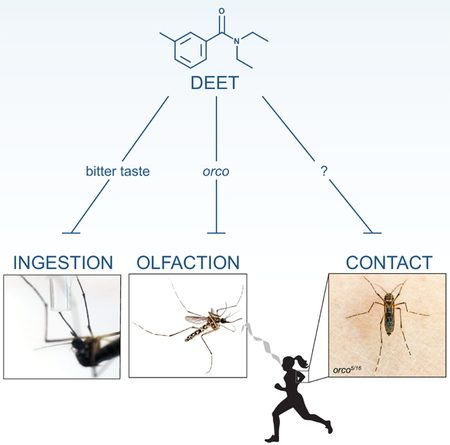
Dennis et al. show that while mosquitoes find DEET and bitters equally distasteful to ingest, only DEET repels mosquitoes on contact. This chemorepellency is mediated by the legs and not the proboscis. These results highlight the multi-modal repellency of DEET and may inform the design of new insect repellents that work on contact.
RESULTS AND DISCUSSION
DEET and Bitter Compounds are Repellent When Ingested, but only DEET is Repellent on Contact
orco mutant mosquitoes are repelled by DEET on contact [12], but the sensory appendages, sensory neurons, and chemosensory receptor genes required for this phenomenon are unknown. To study contact repellency, we used heteroallelic orco5/16 mutant mosquitoes throughout this study to eliminate the olfactory effects of DEET. Wild-type mosquitoes will not approach DEET-treated skin, while orco mutants are attracted to humans and are only repelled after contacting DEET [12]. Here we define “olfactory repellency” as avoidance of volatile DEET that is dependent on orco, “ingestive repellency” as the anti-feedant effect seen after tasting fluid containing an aversive substance, and “contact repellency” as the repellency of a surface, usually but not exclusively human skin.
Classic work in D. melanogaster flies characterized the taste neurons and gustatory receptor (GR) genes responding to a large number of bitter substances that trigger avoidance [13, 14]. Both D. melanogaster flies [9] and Ae. aegypti mosquitoes [15] will reject sucrose tainted with bitter substances. Previous work in Drosophila [9] demonstrated that DEET acts as a bitter tastant when ingested, and that bitter-sensitive taste neurons and bitter GRs are responsible for the anti-feedant effects of DEET. Similar bitter- and DEET-sensitive neurons were identified on the proboscis of the mosquito [10] but their influence on behavior has not been studied.
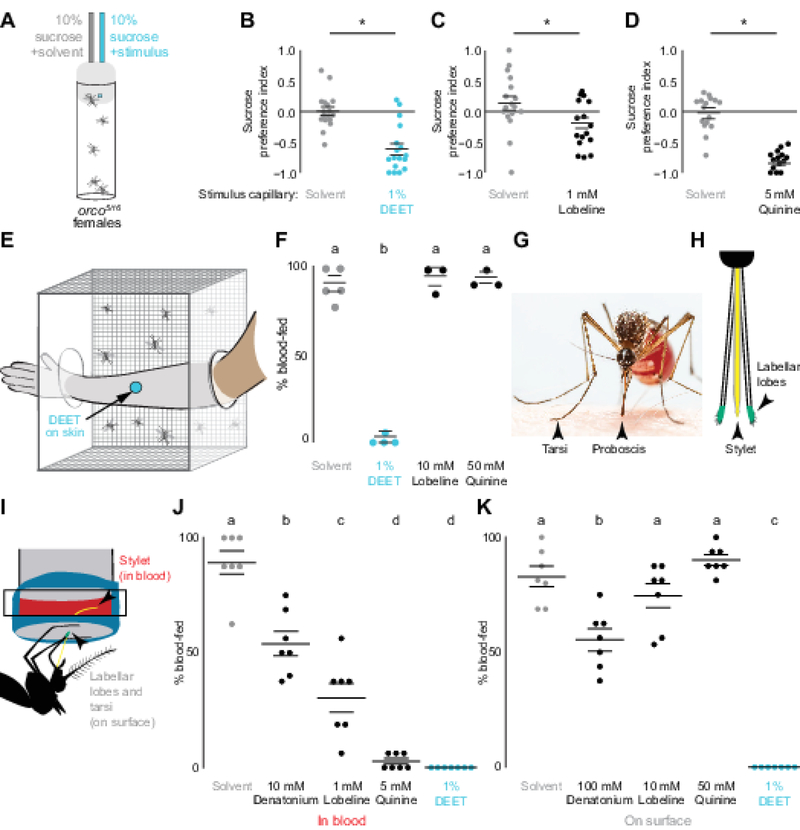
To ask if DEET can inhibit Ae. aegypti mosquito sugar feeding, we used the CAFE assay [15–17] to offer fasted animals a choice between drinking either 10% sucrose or 10% sucrose mixed with either 1% DEET or substances known to be bitter (lobeline or quinine) (Figure 1A). The selection and concentration of bitters throughout this study was guided by classic work from Drosophila [13, 14]. Mosquitoes avoided sucrose containing either bitter tastants or DEET (Figure 1B–D). These data demonstrate that in mosquitoes, as in D. melanogaster flies [9] and Apis mellifera bees [8], DEET and bitter tastants induce avoidance of an otherwise attractive sucrose solution.
Figure 1 |. DEET and Bitter Compounds are Repellent When Ingested, but only DEET is Repellent on Contact.
(A) Mosquito CAFE assay schematic.
(B-D) Inhibition of sucrose ingestion in a two-choice CAFE assay by DEET (B), lobeline (C), or quinine (D) compared to solvent (gray) (N=14–17, n=5 animals/assay).
(E) Arm-in-cage schematic of a DEET-treated arm with a 25 mm circle of accessible skin.
(F) Blood-feeding with the indicated compounds applied to a human arm as in (E) (N=3–5, n=23–25 animals/assay).
(G) Mosquito feeding on a human arm with proboscis and tarsi contacting the skin (Photo: Alex Wild).
(H) Schematic of the sensory appendages of the mosquito proboscis.
(I) Glytube assay schematic highlighting location of appendages during in blood and on surface feeding experiments.
(J-K) Glytube feeding with indicated compounds applied in blood (J) or on surface (K) (N=7, n=15–16 animals/assay).
Horizontal lines in B-D, J, K represent mean ± SEM. Different letters or * indicate statistically significantly distinguishable groups [p<0.05; Student’s t-test (B-D), or one-way ANOVA with Tukey HSD post hoc test (F, J, K)].
See also Video S1.
To ask if the bitterness of DEET accounts for its effective contact chemorepellency on skin, we used a modified arm-in-cage assay [12, 18, 19] (Figure 1E) in which mosquitoes were offered the opportunity to blood-feed on a 25 mm circle of exposed skin treated with solvent, DEET, lobeline, or quinine. For these experiments we increased the concentration of bitters ten-fold. Remarkably, applying either bitter tastant to skin had no effect on mosquito biting and blood-feeding behavior (Figure 1F, Video S1), even though they were delivered at 10-fold higher concentrations than those that deterred sugar feeding. In contrast, DEET applied on the arm provided complete protection (Figure 1F, Video S1).
To reconcile how bitters can be effective anti-feedants in sugar-feeding assays but not in blood-feeding assays, we tested if the delivery of the bitter tastant is the salient difference between the two assays. Mosquitoes contact the skin surface with the proboscis and the terminal segments of the leg, called the tarsi (Figure 1G). To bite a human arm, a mosquito must first saw through the skin and insert a needle-like appendage, the stylet, under the skin (Figure 1H). Therefore, only the stylet is in direct contact with the blood, while the labellar lobes of the proboscis and the tarsi remain on the surface of the skin (Figure 1G–I). We hypothesized that bitter tastants mixed into blood might deter ingestion but would not interfere with blood-feeding if presented on contact only.
To test this hypothesis, we used a Glytube feeding assay (Figure 1I) [20]. The Glytube assay uses a piece of Parafilm as a skin-substitute to cover a small amount of warmed sheep blood. This allows us to deliver DEET or bitters either mixed into the blood or presented on the surface of the Glytube, an experiment not feasible to conduct with live human subjects. We observed that the bitters denatonium, lobeline, and quinine as well as DEET all inhibited blood-feeding when mixed into blood in the Glytube assay. While denatonium had modest activity as an anti-feedant, quinine and DEET strongly inhibited the ingestion of blood (Figure 1J). When the bitter substances were applied to the surface of the Glytube, neither lobeline nor quinine had any effect on blood-feeding, and denatonium showed only a modest reduction. In contrast, DEET completely inhibited blood-feeding on contact (Figure 1K). These results support our conclusions from the arm-in-cage experiments in Figure 1E–F and agree with recent findings from Culex quinquefasciatus mosquitoes, which demonstrated that animals spent less time feeding on Parafilm-covered blood-soaked cotton balls if DEET was mixed into the blood [11]. These data support the hypothesis that DEET is bitter by ingestion but its bitterness per se does not explain its highly effective contact chemorepellency.
The Legs, not the Proboscis, are Required for Contact DEET Repellency
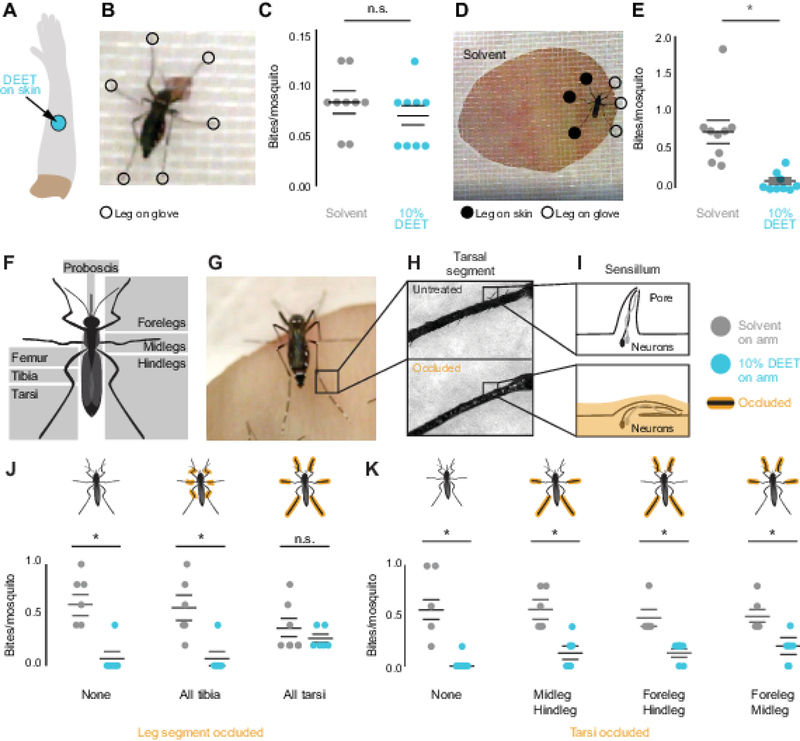
Insects have chemosensory neurons on their mouthparts, ovipositor, legs, and even their wings [21]. In search of the sensory appendages responsible for DEET contact repellency, we focused on the proboscis and legs because they are the primary appendages that contact the skin during landing (Figure 1G). The legs are particularly interesting candidate appendages because many insects use their legs to evaluate food sources [22, 23]. Additionally, in D. melanogaster, gustatory neurons in the legs can respond differently to tastants than neurons in the proboscis [14]. To test if the proboscis is sufficient to mediate contact DEET repellency, we modified the arm-in-cage assay to restrict the area of skin available for the mosquitoes to contact (Figure 2A–B). The ~1.5 mm diameter circle of exposed skin we used in this assay is smaller than the distance between a mosquito’s forelegs, and it therefore cannot touch the skin with both proboscis and legs at the same time (Figure 2B–C). In this assay, mosquitoes blood-fed equally on solvent- and DEET-treated arms, demonstrating that the proboscis alone is not sufficient for DEET repellency (Figure 2C). In contrast, when we enlarged the diameter of exposed skin so that both the legs and the proboscis could contact the skin (Figure 2D), DEET remained an effective contact repellent (Figure 2E). These data provide evidence that the proboscis is not sufficient to deter mosquitoes from biting DEET-treated human skin.
Figure 2 |. The Tarsi of the Legs, not the Proboscis, are Required for Contact DEET Repellency.
(A) Schematic of a DEET-treated arm with a 25 mm circle of accessible skin.
(B) Video still of a mosquito feeding 1.5 mm circle of accessible skin treated with DEET.
(C) Average number of biting events per mosquito on solvent-treated or DEET-treated skin (N=9 assays, 23–25 animals/assay).
(D) Video still of a mosquito feeding on a solvent-treated arm through a 25 mm circle of accessible skin, with positions of legs manually scored.
(E) Mosquito biting events on solvent-treated or DEET-treated skin (N=9 assays, 23–25 animals/assay).
(F) Schematic of mosquito leg anatomy and the proboscis.
(G) Video still of a mosquito on a human arm, highlighting the tarsi.
(H) Examples of untreated (top) or UV glue occluded (bottom) tarsal segments.
(I) Schematic of tarsal sensillum after occlusion by UV glue.
(J-K) Mosquito biting events on solvent-treated or DEET-treated arms. Cartoon mosquitoes indicate which appendages were occluded (N=6 assays, 5 animals/assay).
Horizontal lines in C, E, J, and K represent mean ± SEM. Statistical significance was assessed with Student’s t-test (* p<0.05; n.s., not significant).
See also Figure S1.
We next investigated if the legs are required to sense DEET on skin. The tarsal segments of the leg are covered in sensory hairs called sensilla, which have pores that allow tastants to enter and activate sensory neurons [24] (Figure 2F–I). We carried out experiments that asked whether some or all legs mediate DEET contact repellency. Initial experiments to surgically remove all tarsi were uninterpretable because the tarsi are required to produce the necessary force and leverage to pierce the skin [25]. To disrupt tarsal chemosensation without removing the tarsi, we coated them with UV-curing glues which have been used previously to occlude sensilla in taste organs [26] and antennae [27] in D. melanogaster flies (Figure 2H–I).
When all tarsi were occluded by gluing, mosquitoes were no longer repelled by DEET-treated skin and bit DEET- and solvent-treated arms equally (Figure 2J). Animals that were sham-treated or with their upper legs (tibia), glued were still repelled by DEET on contact (Figure 2J), suggesting that the tarsi are necessary for contact DEET repellency. While observing the animals interact with these small areas of available skin surface, we noticed that they did not always contact the skin with all six legs (Figure 2D, 2G). We therefore asked if any pair of tarsi was dispensable or required for contact DEET repellency. Leaving any pair of tarsi unoccluded was sufficient to decrease biting events (Figure 2K), suggesting that any pair of tarsi is sufficient to deter blood feeding on DEET-treated arms. Scoring of individual landing events indicated that, in the rare cases where an animal bit a DEET-treated arm, they often contacted the skin with only occluded legs and the proboscis (Figure S1A–D) and biting events were usually brief (Figure S1E–H). We speculate that the chemosensory neurons and receptors that sense DEET are present in tarsi on all six legs.
CONCLUSIONS
DEET is a small, synthetic molecule that is the world’s most effective and widely used insect repellent [28]. Developed in World War II to protect soldiers threatened by mosquito-borne diseases such as malaria and yellow fever, DEET has been in civilian use for over 70 years [29]. It protects humans against bites from animals across vast evolutionary distances, including land leeches [30], ticks [31], and mosquitoes [32, 33]. Although highly effective, it has several undesirable properties that limit its practical use. It is oily on the skin and must be reapplied liberally at very high concentrations on all areas of exposed skin every 6 hours. This is impractical in the tropical zones where pathogen-infected mosquitoes are most dangerous. Despite various efforts to improve upon DEET, it remains the gold standard for personal protection. Remarkably, its mechanism of action is still incompletely understood, and this gap in our knowledge prevents the rational design of new highly effective molecules that address the deficiencies of DEET.
Our work highlights the multi-modal action of DEET [2, 3, 5–11]. In addition to its role in olfactory avoidance, DEET both inhibits ingestion and acts as a potent contact chemorepellent in the mosquito. We show here that contact chemorepellency is mediated by the tarsal segments of the leg and not by the proboscis. Substances that taste bitter to flies and mosquitoes when ingested are ineffective in repelling mosquitoes on contact. In D. melanogaster, DEET activates specific bitter-tuned gustatory neurons and bitter GRs are required for DEET to inhibit ingestion of sucrose agar [9], but these experiments could not disambiguate the ingestive and contact effects of DEET. Several mechanisms could explain the tarsal contact DEET chemorepellency observed in mosquitoes. DEET could activate bitter GRs that are more selectively tuned or more sensitive to DEET than conventional bitters. Such DEET-selective neurons could be hardwired into a labeled line aversion pathway that triggers stronger avoidance than bitters. Alternatively, DEET could act on a non-GR class of receptors selectively expressed in tarsi. Indeed, tissue-specific RNA-seq data indicate that many chemosensory receptors including IRs and pickpocket and TRP ion channels are expressed in the legs, several of which are not detected in the proboscis [34].
These results demonstrate the value of studying mechanisms across multiple species. Although there is strong genetic and anatomical conservation in chemosensory systems across insects, mosquitoes have likely evolved specialized sensing mechanisms relevant to their lifestyle as blood-feeding insects. Drosophila walk on their food while they ingest it, so that the same tastants are stimulating tarsal neurons and ingestive neurons in the proboscis. In contrast, female mosquitoes walk on the surface of the skin but must puncture the skin to drink blood, so that different tastants are stimulating tarsal and ingestive neurons. These anatomical and behavioral differences may have driven differences in chemosensory gene expression, function, and neural circuits in these two insects. Moreover, these specialized mechanisms may reveal potential targets leading to improvements in the next generation of insect repellents.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Leslie Vosshall (leslie.vosshall@rockefeller.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mosquito rearing and maintenance
All experiments in this study used female Aedes aegypti orco5/16 heteroallelic mutants, which were generated by crossing homozygous orco5/5 and orco16/16 mutants and collecting F1 progeny (NR-443377 and NR-44378, BEI Resources) [12]. Mosquitoes were reared at 25–28°C, 70–80% relative humidity with a photoperiod o f 14 hours light:10 hours dark (lights on at 7 AM) as previously described [12]. Eggs were hatched in deoxygenated, deionized water containing powdered Tetramin tablets (fish food) (Tetra; 16110M, Pet Mountain). Larvae were fed additional Tetramin until pupation. Pupae were placed in a small cup of deionized water, moved to a 30 cm3 mosquito cage (211261, Bugdorm), and allowed to eclose. Mosquitoes were co-housed and females were presumed to be mated by the time of the experiments. Animals were provided with unlimited access to 10% sucrose (57-50-1, Thermo Fisher; weight:volume in deionized water), and blood-fed on mice for routine strain maintenance. Blood-feeding on mice was approved and monitored by The Rockefeller University Institutional Animal Care and Use Committee (protocol 15772). Human subjects provided written, informed consent to participate in experiments, and these procedures were approved and monitored by The Rockefeller University Institutional Review Board (protocol LV-0652).
METHOD DETAILS
Mosquito behavior experiments
All behavioral experiments were carried out with 7–14 day-old animals that had not previously taken a blood-meal. All assays except the Glytube experiments in Figure 1J–K were carried out at Zeitgeber time (ZT) 6-ZT10 at 25–28°C and 70–80% relative humidity. Assays in Figure 1J–K were carried out at ZT9-ZT13 at 24–26°C and 80–90% relative humidity. Unless otherwise stated, cold anesthesia was carried out by working with animals in a 4° C cold room. Blood-feeding was scored by identifying an observable stretching of the pleural membrane in the midgut and red coloration from ingested blood in the abdomen. No partially blood-fed mosquitoes were observed in arm-in-cage assays in Figure 1F or the on-surface Glytube experiments in Fig 1K. Not all animals in the in blood Glytube experiments in Figure 1J fed to repletion, but animals could be unambiguously scored as blood-fed or non-blood-fed by visual inspection.
Two-choice CAFE feeding assay
Animals were sexed and sorted under cold anesthesia and fasted for 18–20 hours with access to water. This assay was adapted for the mosquito from the Drosophila melanogaster CAFE assay [16] as described previously [15]. At the start of each trial, five fasted mosquitoes were transferred by mouth pipette to a narrow Drosophila polystyrene vial (32–116, Genesee Scientific) with two 5 μL calibrated glass capillaries (53432–706, VWR) embedded in cotton flugs (49–102, Genesee Scientific) and barely protruding from the bottom of the flug surface. The top surface of the flug was marked with a Sharpie to indicate control and stimulus sides. A small piece of red tape (89097–932, VWR) was affixed to the bottom surface of the flug with capillaries protruding from it, to provide visual contrast that increased participation in the assay [15]. One capillary served as the control, containing 10% sucrose (weight:volume) in deionized water supplemented with 1% ethanol solvent (E7023, Millipore Sigma). The stimulus capillary contained 10% sucrose supplemented with one of the following chemicals: 1% DEET (CID 24893319; D100951, Millipore Sigma), 1 mM (−)-lobeline hydrochloride (CID 101615; 141879, Millipore Sigma), or 5 mM quinine (CID 3034034; 22620, Millipore Sigma). These were prepared from 100X stock solutions in ethanol for bitter tastants, or 50% DEET in ethanol, such that the final concentration of ethanol in 10% sucrose was 1%. After four hours, the remaining liquid in all capillaries was measured manually in millimeters, by aligning a metric ruler to the tip of the capillary and measuring the height of the liquid meniscus. Mosquito-less vials with 2 capillaries filled with 10% sucrose and 1% ethanol served as evaporation controls. Eight evaporation vials were used each day. The experimenter was not aware of the identity of the stimulus in a given CAFE assay, because a volunteer anonymized them prior to the experiment. An average evaporation amount for each day of experiments was calculated (EVAP) by first calculating an average of the evaporation from the two control capillaries in each vial, and then averaging across all evaporation control vials on that day. For each test vial, the reduction in liquid level was recorded for the 10% sucrose capillary (CONTROL) and the 10% sucrose + stimulus capillary (STIMULUS). The preference index was calculated as follows: [(STIMULUS – EVAP) – (CONTROL – EVAP)] / [(STIMULUS – EVAP) + (CONTROL – EVAP)]. Vials were excluded if any of the 5 animals died during the assay.
Glytube blood-feeding assay
Animals were sexed and sorted into groups of 15–16 under cold anesthesia and fasted for 21–25 hours with access to water. For each condition, 3 mL of defibrinated sheep blood (DSB500, Hemostat Laboratories) was warmed in a 15 mL polystyrene Falcon conical tube (352095, Corning) for 15 minutes using a 42°C bead bath (A1254302, Fisher Scientific). Upon removal from the heat bath, 300 μL of a 20 mM ATP (A3377, Millipore Sigma) stock solution in 25 mM NaHCO3 was added for a final ATP concentration of 1.8 mM. ATP was mixed into the blood by vigorous shaking by hand. Because additional volumes of test substances were added for the in blood Glytube experiments, the final ATP concentration in these experiments was 1.62 mM. Aliquots of ATP were stored at −20° C and thawed at room temperature approximately 20 minutes before each trial. 1.5 mL defibrinated sheep blood was then loaded into Glytube membrane feeders as described [20]. Bitter compounds or DEET were applied by dipping the Glytube into solution for on surface experiments or were added directly to blood prior to being loaded in Glytube for in blood experiments (see below for details). The per cent blood-fed was calculated by counting the number of fed mosquitoes divided by the total number of mosquitoes, multiplied by 100.
Assembled Glytubes were delivered to mosquitoes within 4 minutes of removal of sheep blood from the heat bath. CO2 to activate mosquitoes was delivered immediately prior to the start of the assay via human breath. Glytubes were placed directly on the mesh tops (98315K58, McMaster Carr) of cardboard cups (KH16A-J8000, Webstaurant) housing each group of mosquitoes. Glytubes were flush with the surface of the mesh, easily accessible for the mosquitoes to touch the Parafilm (Bemis™ Parafilm M™ Laboratory Wrapping Film, 13-374-10, Fisher Scientific) and puncture it for blood-feeding. Animals were allowed access to the Glytube for 15 minutes, after which the Glytubes were removed, and cups were moved to a 4°C refrigerator for cold anesthesia. Animals scored for blood-feeding status on wet ice. The experimenter was aware of the stimuli as the experiment was not anonymized. All conditions were performed once per experimental day, in a randomized sequence with independent groups of mosquitoes.
On surface Glytube experiments
The following substances were applied to the Parafilm for the on surface Glytube experiments: 50 mM quinine (CID 3034034; 22620, Millipore Sigma), 100 mM denatonium benzoate (CID 19518; D5765, Millipore Sigma), 10 mM (−)-lobeline hydrochloride (CID 101615; 1077, TOCRIS), and 1% DEET. The bitter solutions were prepared as powders dissolved in ethanol (CID 329799002, BP2818500, Fisher Scientific). Liquid 100% DEET was diluted to 1% in ethanol (volume:volume). The solvent condition used 100% ethanol. Test solutions were prepared the day of the experiment in 15 mL or 50 mL polystyrene Falcon conical tubes (352095 and 352070, Corning) at room temperature. Prior to start of a trial, 4–5 mL of bitter solution, DEET, or solvent was dispensed into a 60 × 15 mm Petri dish (08-757-13A, Fisher Scientific) for dipping the Glytube to coat the surface of the Parafilm. The Parafilm surface of the Glytube was carefully touched to the surface of the bitter solution. This was done to avoid the solution reaching above the edge of the Parafilm and potentially contaminating the blood. A paper towel was used to wick off any excess liquid but did not touch to the Parafilm surface directly. Glytubes were kept vertical, with the Parafilm surface facing down, to avoid spilling of the blood within the Glytube. The Glytube was delivered to mosquitoes immediately after dipping by placing it on top of mesh top of the cup containing mosquitoes.
In blood Glytube experiments
In blood Glytube experiments used 90% sheep blood solution (as prepared above), 1.62 mM ATP, and 1% ethanol as the solvent control condition, with bitters or DEET added as test solutions. Ultrapure water (Milli-Q Advantage A1, Millipore) was used as a diluent as described below. 10 mM denatonium benzoate, and 1 mM (−)-lobeline hydrochloride were prepared as a 10x stock solution in 10% ethanol (volume:volume, in ultrapure water) and added directly to the blood solution. 5 mM quinine was prepared as a 100x stock solution in 100% ethanol. Glytubes for quinine in blood conditions were prepared by first adding 9% ultrapure water (by final volume) followed by quinine in ethanol. Glytubes for DEET in blood conditions were prepared by first adding ethanol and ultrapure water to a final concentration of 1% and 8%, respectively, followed by undiluted liquid 100% DEET to a final concentration of 1%. Test solutions were prepared the day of the experiment and kept at room temperature. Solutions were added to pre-heated blood, then shaken vigorously by hand before being pipetted into the Glytube cap and delivered immediately to mosquitoes.
Human blood-feeding and biting assays
Standard arm-in-cage biting assays [18, 19] were carried out with modifications as previously described [12] and additional modifications in each section below. To prepare an arm, three horizontal lines were drawn approximately 48, 50, and 52 mm above the wrist in ethanol-soluble ballpoint pen ink. 0.5 mL of either solvent or a test substance in solvent (lobeline, quinine, or DEET) was added to the or lower forearm of a human volunteer (27-year-old female) before donning the glove. The test substance was applied above (for upper forearm) or below (for lower forearm) the middle ballpoint pen line by pipetting it directly onto the skin. The test substance was then evenly and gently spread onto the skin using a gloved finger, causing the closest ballpoint pen ink line (top for upper forearm, bottom for lower forearm) to be smeared, while leaving the middle line unsmeared. This prevented cross-contamination of the skin areas used for experiments. After applying the test substance to the appropriate area, an elbow-length latex glove (19-668-001, Fisher Scientific) with a hole cut into it was donned. The hole exposed a small area of solvent-treated or test substance-treated skin. The holes in the gloves corresponded to an area above the middle ballpoint pen line (upper forearm) or below the middle ballpoint pen line (lower forearm).
The arm-in-cage blood feeding assays were not filmed, because they were endpoint assays in which blood-feeding was scored. All other assays described in this section used a Canon EOS60D camera at 60 frames/second directed at the arm. The camera lens was inserted into a cage through a mesh sleeve opening. An arm was either pressed against the opposing side of the cage for the constrained feeding access assay or inserted into the cage through a mesh sleeve opening on the wall adjacent to the camera. The arm was positioned in front of the camera, through the middle of the cage, as drawn in Figure 1E. The per cent blood-fed was calculated by counting the number of fed mosquitoes divided by the total number of mosquitoes, multiplied by 100.
Videos were anonymized in groups of 8 or 12 by a volunteer who changed the names of the videos. The videos were then scored, frame by frame, to record the number of skin contacts and bites. A contact was defined as when a mosquito landed on the skin, contacting the skin with at least one tarsi or proboscis while the wings had stopped moving. If no contacts were observed, the video was discarded. This was rare (<2% of videos). A bite was scored if the following three criteria were met (1) the proboscis was in contact with the skin (2) the mosquito forelegs and midlegs were stationary (3) the mosquito head did not move, and then a characteristic sawing motion was visible. Whenever possible, the bite was confirmed by noting that the skin reddened, although some bites occurring at the very end of an assay could not be confirmed in this way. Bite scoring did not require visual detection of blood in the abdomen, as this was often difficult either because of the duration of the feeding event, for instance a bite commencing at 9:45 of a 10:00 video, or if multiple animals were present and blocked the view of the abdomen of the neighboring mosquito.
Arm-in-cage blood-feeding assays
A group of 25 mosquitoes was released into a 30 cm3 mosquito cage and given five minutes to acclimate. The gloved arm was then placed in the cage for ten minutes after which the arm was removed and cage moved to a 4° C cold room to anesthetize the animals. Animals were scored as blood-fed or non-blood-fed based by visual inspection of the abdomen. No external CO2 was added to these cages but assays were carried out in close proximity to a breathing human. These assays were anonymized by re-labeling the test substances before application to the arm. These assays were pseudo-randomized such that the stimuli were provided in a different order each day, and pseudorandomized in their location on the upper left, upper right, lower left, or lower right forearm. Also, a solvent control was included each day of experimentation.
Constrained feeding access assay
This assay is a modification of the arm-in-cage assay, where the gloved human arm exposing either 25 mm or 1.5 mm of skin is instead pressed against the mesh on the outside of the cage. By decreasing the surface area that the mosquitoes could explore before finding the hole in the glove, participation in the small hole (1.5 mm) trials was increased. These assays were not anonymized and were pseudo-randomized such that the stimuli were provided in a different order each day, and a solvent control was included each day of experimentation. The videos were anonymized before manual annotation by re-naming the files. Bites/mosquito was calculated for each video by dividing the number of animals biting by the number of animals in the assay.
Arm-in-cage mosquito leg occlusion biting assays
Animals were transferred from cages into cups in groups of five using a mouth aspirator and anesthetized by placing the cups on wet ice. Individual mosquitoes were transferred onto top of a 10 cm Petri dish filled with wet ice such that the mosquito was kept cool throughout the procedure but did not contact the ice directly. Working under a dissecting microscope, tarsi were glue-occluded by inserting them one at a time into the narrow end of a 1 mL pipette tip (CLS4868, Millipore Sigma) containing 200–500 μL UV curing glue (KOA 300–1, Kemxert), coating the legs, which were then removed from the pipette tip and cured with a 405 nm 5 mW laser pointer (QQ-Tech) for 20 seconds with the laser pointer held approximately 25 mm away from the tip of the tarsi, and pointed toward the abdomen to illuminate the whole tarsi. Sham-treated controls were handled identically with the exception that the pipette tip was empty, so no glue was applied. Tibia were glued by slowly applying UV glue with a 200 μL pipette tip (21-377-354, Fisher Scientific) until coated, then cured for 20 seconds as described above. The process for each animal took 2–5 minutes and they were returned to the soup cup with a mesh lid to recover. Animals with the same treatment were housed in groups of 5 females for 18–24 hours with access to water at 25–28°C and 70–80% re lative humidity. If any animals died overnight, that group of animals was discarded. This was a rare occurrence. Bites/mosquito was calculated for each video by dividing the number of animals biting by the number of animals in the assay.
QUANTIFICATION AND STATISTICAL ANALYSIS
R version 3.3.2 Sincere Pumpkin Patch (CRAN) was used for all statistical analyses. Statistical details including exact values of N and what N represents are indicated in the figure legends and any calculations are defined in the method details. Significance was defined as p<0.05. Sample sizes were estimated by a power analysis on pilot data, with the exception of occlusion experiments and Glytube feeding experiments, which were based on sample sizes of previous studies. Exclusion criteria, anonymization, and randomization for each behavioral assay are defined in the method details.
Supplementary Material
Data S1. Raw Data, Related to Figure 1 and 2 and Figure S1. This Excel file contains all of the raw data used to generate the figures in the paper
Video S1. Arm-in-cage landing with bitters and DEET, Related to Figure 1F
This video shows examples of mosquitoes participating in arm-in-cage experiments with various substances applied to the skin. Female orco5/16 heteroallelic mutants were filmed for 10 minutes interacting with a human arm treated with 100% ethanol solvent, 50 mM quinine, 90 mM lobeline, or 1% DEET. Representative 60 second clips were taken. Video is played at actual speed.
HIGHLIGHTS.
DEET and bitters inhibit Aedes aegypti female mosquito sugar ingestion
Only DEET completely prevents blood-feeding on contact
Repellency of DEET on skin contact is mediated by the tarsal segments of the legs
Any pair of legs can sense DEET to prevent mosquito biting
Acknowledgments
We thank Josie Clowney, Matthew DeGennaro, Itzel Ishida, Xin Jin, Kevin Lee, and members of the Vosshall lab for discussion and comments on the manuscript, Matthew DeGennaro for early discussions, Vineeta Reddy for technical assistance with experiments in Figure 1B–D, Tim Dennis for anonymizing all videos before analysis in Figure 2, and Christoph Rainer for assistance in production of Video S1. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number F31DC014222 awarded to E.J.D. Supported in part by grant # UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. L.B.V. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
DATA AND SOFTWARE AVAILABILITY
Software and custom scripts used for statistical analysis, plotting, and manual video annotation are listed in the Key Resources Table. All data in the paper are available in Data S1, with the exception of raw video files, which are available upon request.
REFERENCES
- 1.DeGennaro M (2015). The mysterious multi-modal repellency of DEET. Fly (Austin) 9, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ditzen M, Pellegrino M, and Vosshall LB (2008). Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Pitts RJ, Bohbot JD, Jones PL, Wang G, and Zwiebel LJ (2010). Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, and Vosshall LB (2011). A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed Z, and Leal WS (2008). Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci U S A 105, 13598–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, and Vosshall LB (2013). orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klun JA, Khrimian A, and Debboun M (2006). Repellent and deterrent effects of SS220, Picaridin, and Deet suppress human blood feeding by Aedes aegypti, Anopheles stephensi, and Phlebotomus papatasi. J Med Entomol 43, 34–39. [DOI] [PubMed] [Google Scholar]
- 8.Abramson CI, Giray T, Mixson TA, Nolf SL, Wells H, Kence A, and Kence M (2010). Proboscis conditioning experiments with honeybees, Apis mellifera caucasica, with butyric acid and DEET mixture as conditioned and unconditioned stimuli. J Insect Sci 10, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Kim SH, and Montell C (2010). Avoiding DEET through insect gustatory receptors. Neuron 67, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanford JL, Shields VDC, and Dickens JC (2013). Gustatory receptor neuron responds to DEET and other insect repellents in the yellow-fever mosquito, Aedes aegypti. Naturwissenschaften 100, 269–273. [DOI] [PubMed] [Google Scholar]
- 11.Lu W, Hawang JK, Zeng F, and Leal WS (2017). DEET as a feeding deterrent. PLoS One 12, e0189243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, and Vosshall LB (2013). orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, and Carlson JR (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling F, Dahanukar A, Weiss LA, Kwon JY, and Carlson JR (2014). The molecular and cellular basis of taste coding in the legs of Drosophila. J Neurosci 34, 7148–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corfas RA, and Vosshall LB (2015). The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. Elife 4, e11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, and Benzer S (2007). Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A 104, 8253–8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liesch J, Bellani LL, and Vosshall LB (2013). Functional and genetic characterization of neuropeptide Y-like receptors in Aedes aegypti. PLoS Negl Trop Dis 7, e2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreck CE (1977). Techniques for the evaluation of insect repellents: a critical review. Annu Rev Entomol 22, 101–119. [DOI] [PubMed] [Google Scholar]
- 19.Logan JG, Stanczyk NM, Hassanali A, Kemei J, Santana AE, Ribeiro KA, Pickett JA, and Mordue Luntz AJ (2010). Arm-in-cage testing of natural human-derived mosquito repellents. Malar J 9, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa-da-Silva AL, Navarrete FR, Salvador FS, Karina-Costa M, Ioshino RS, Azevedo DS, Rocha DR, Romano CM, and Capurro ML (2013). Glytube: a conical tube and parafilm M-based method as a simplified device to artificially blood-feed the dengue vector mosquito, Aedes aegypti. PLoS One 8, e53816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stocker RF (1994). The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275, 3–26. [DOI] [PubMed] [Google Scholar]
- 22.Minnich DE (1922). The chemical sensitivity of the tarsi of the red admiral butterfly, Pyrameis atalanta Linn. . J Exp Zool 35, 57–81. [Google Scholar]
- 23.Meunier N, Marion-Poll F, Rospars JP, and Tanimura T (2003). Peripheral coding of bitter taste in Drosophila. J Neurobiol 56, 139–152. [DOI] [PubMed] [Google Scholar]
- 24.McIver S, and Siemicki R (1978). Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae). J. Morphol 155, 137–156. [DOI] [PubMed] [Google Scholar]
- 25.Jones JC, and Pilitt DR (1973). Blood-feeding behavior of adult Aedes aegypti mosquitoes. Biol. Bull 145, 127–139. [DOI] [PubMed] [Google Scholar]
- 26.Olsen SR, and Wilson RI (2008). Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasserman S, Salomon A, and Frye MA (2013). Drosophila tracks carbon dioxide in flight. Curr Biol 23, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz TM, Miller JH, and Hebert AA (2008). Insect repellents: historical perspectives and new developments. J Am Acad Dermatol 58, 865–871. [DOI] [PubMed] [Google Scholar]
- 29.McCabe ET, Barthel WF, Gertler SI, and Hall SA (1954). Insect Repellents. III. N, N-diethylamides. J. Org. Chem 19, 493–498. [Google Scholar]
- 30.Tawatsin A, Thavara U, Chansang U, Chavalittumrong P, Boonruad T, Wongsinkongman P, Bansidhi J, and Mulla MS (2006). Field evaluation of deet, Repel Care, and three plant based essential oil repellents against mosquitoes, black flies (Diptera: Simuliidae) and land leeches (Arhynchobdellida: Haemadipsidae) in Thailand. J Am Mosq Control Assoc 22, 306–313. [DOI] [PubMed] [Google Scholar]
- 31.Evans SR, Korch GW Jr., and Lawson MA (1990). Comparative field evaluation of permethrin and deet-treated military uniforms for personal protection against ticks (Acari). J Med Entomol 27, 829–834. [DOI] [PubMed] [Google Scholar]
- 32.Bernier UR, Furman KD, Kline DL, Allan SA, and Barnard DR (2005). Comparison of contact and spatial repellency of catnip oil and N,N-diethyl-3-methylbenzamide (deet) against mosquitoes. J Med Entomol 42, 306–311. [DOI] [PubMed] [Google Scholar]
- 33.Thavara U, Tawatsin A, Chompoosri J, Suwonkerd W, Chansang UR, and Asavadachanukorn P (2001). Laboratory and field evaluations of the insect repellent 3535 (ethyl butylacetylaminopropionate) and deet against mosquito vectors in Thailand. J Am Mosq Control Assoc 17, 190–195. [PubMed] [Google Scholar]
- 34.Matthews BJ, McBride CS, DeGennaro M, Despo O, and Vosshall LB (2016). The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics 17, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Raw Data, Related to Figure 1 and 2 and Figure S1. This Excel file contains all of the raw data used to generate the figures in the paper
Video S1. Arm-in-cage landing with bitters and DEET, Related to Figure 1F
This video shows examples of mosquitoes participating in arm-in-cage experiments with various substances applied to the skin. Female orco5/16 heteroallelic mutants were filmed for 10 minutes interacting with a human arm treated with 100% ethanol solvent, 50 mM quinine, 90 mM lobeline, or 1% DEET. Representative 60 second clips were taken. Video is played at actual speed.