Figure 3.
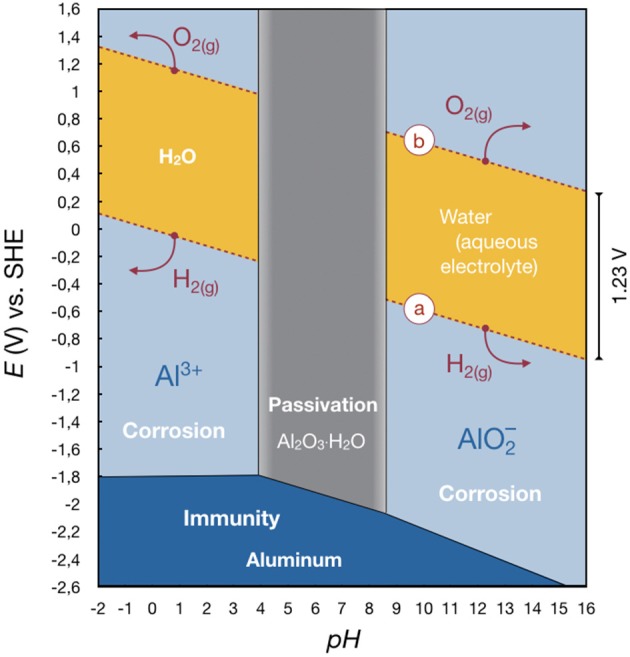
Pourbaix diagram of aluminum in water at 25°C showing its corrosion behavior. It depicts the basic oxidation/reduction reactions for aluminum in aqueous systems. Outside the yellow region, water breaks down, not the metal. It can be seen that a secondary aluminum-ion battery with an aluminum metal as negative electrode based on an aqueous system will not be possible since the aluminum cannot be plated both at low and high pH. It cannot be solved in a medium pH, as well. Therefore, just primary battery systems can be realized (cf. section Aqueous or Primary Aluminum Battery). Redrawn from Deltombe and Pourbaix (1958), Vargel (2004), and Ashby and Jones (2012).
