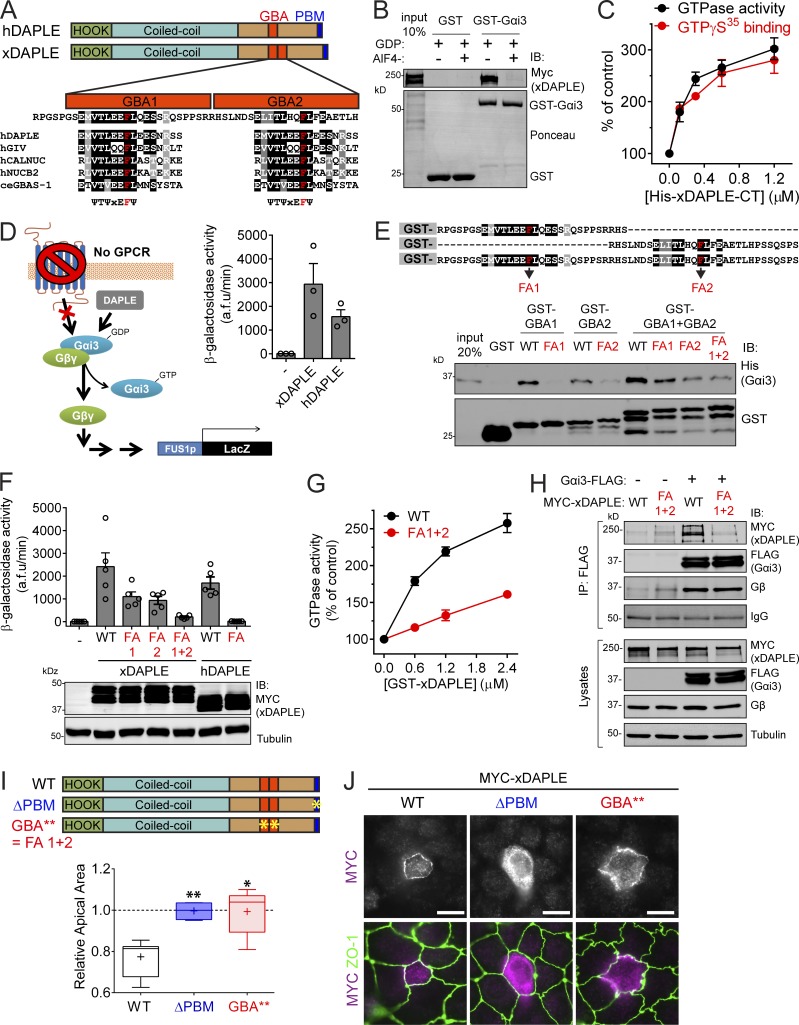
Figure 5.
xDAPLE promotes G protein signaling and apical cell constriction via two GBA motifs in tandem. (A) Comparison of domain composition of hDAPLE and xDAPLE, and alignment of xDAPLE GBA1 and GBA2 with other GBA motifs. ce, C. elegans; h, Homo sapiens. (B) Full-length xDAPLE from HEK293T cell lysates binds to immobilized GST-Gαi3 when the G protein is loaded with GDP (inactive), but not when it is loaded with GDP-AlF4−. (C) Steady-state GTPase (black) and GTPγS binding (red) experiments showing that purified His-xDAPLE-CT accelerates nucleotide exchange of purified His-Gαi3. Results are the average ± SEM of n = 3 experiments. (D) xDAPLE-CT and hDAPLE-CT activate G protein signaling in a yeast-based β-galactosidase reporter assay. Diagram on the left depicts the pathway activated by DAPLE in yeast lacking the cognate GPCR and with the endogenous G protein replaced by human Gαi3. Results on the right are the average ± SEM of n = 3 experiments. (E) Protein–protein binding experiment showing that purified His-Gαi3 binds to both GBA1 and GBA2 of xDAPLE. Diagram on the top shows a detail of the sequence of the purified GST-fused xDAPLE constructs used in the experiment and the position of FA point mutations (in red). (F) G protein activity assays in yeast (as in D) show that both GBA1 and GBA2 have to be mutated simultaneously to abolish xDAPLE-mediated activation. Results are the average ± SEM of n = 5 experiments. (G) Steady-state GTPase experiments showing that activation of purified His-Gαi3 by GST-xDAPLE FA1+2 (red) is impaired compared GST-xDAPLE WT (black). Results are the average ± SEM of n = 3 experiments. Basal activity = 0.16 mol Pi/mol Gαi3/15 min. (H) Coimmunoprecipitation (IP) experiments showing that xDAPLE WT, but not xDAPLE FA1+2 mutant, binds to Gαi3-FLAG when expressed in HEK293T cells. Immunoblots (IB) of the FLAG IPs are shown on the top and equal aliquots of the starting lysates used for it are shown on the bottom. (I) Box-and-whiskers plots (minimum to maximum) for the quantification of relative apical area show xDAPLE ΔPBM and xDAPLE GBA** (FA1+2) mutants fail to promote apical constriction in MDCK cells compared with xDAPLE WT. Results are from n = 4–9 independent experiments. *, P < 0.05; **, P < 0.01 using the Mann–Whitney U test. (J) Fluorescence microscopy pictures of MDCK cells sparsely expressing the indicated MYC-xDAPLE constructs and costained for MYC (magenta) and ZO-1 (green) show that xDAPLE GBA** mutant is enriched at apical junctions like WT, while xDAPLE ΔPBM mutant is not. Scale bars, 10 µm.