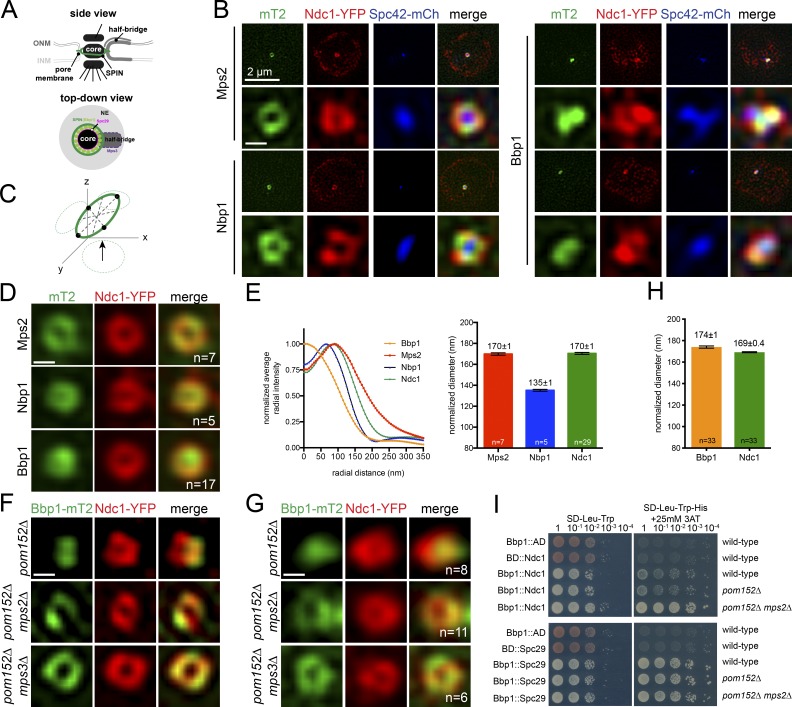
Figure 1.
Radial distribution of SPIN components at the SPB. (A) SPB schematic showing the core, SPIN, and half-bridge based on a side-on or top-down view of the SPB. Through Bbp1 binding to Spc29, the SPIN is thought to form a pore for SPB insertion and anchorage. (B) Representative SIM images of nuclei (bar, 2 µm) with Ndc1-YFP (red) and Spc42-mCherry (blue) to detect the SPB ring and core, respectively, along with the indicated protein tagged with mT2 (green; SLJ11171, Mps2; SLJ10898, Nbp1; SLJ10635, Bbp1). Ndc1-YFP is also present at NPCs (Chial et al., 1998). (C and D) As shown in C, averaged images (D) were generated by realigning multiple SPB rings as indicated (n) in 3D (see Fig. S1, A and B). (E) Fluorescence profiles of SPIN components from the center of the SPB outwards, based on the projections in D. Average ring diameter was determined in aligned images based on the center of Gaussian fits of fluorescence intensity. Because Ndc1-YFP diameter varied by ≤20 nm between different strain isolates, values were normalized using Ndc1-YFP values. Errors were determined by Monte Carlo analysis. (F) Localization of Ndc1-YFP (red) and Bbp1-mT2 (green) in asynchronously grown pom152Δ (SLJ12302), pom152Δ mps2Δ (SLJ10998), and pom152Δ mps3Δ (SLJ10534) strains by SIM. (G and H) Averaged images were generated as in D, and average ring diameter was determined from the mutants, as in E. (I) Pairwise protein interactions between Bbp1 fused to the Gal4-binding domain (BD) and Ndc1 or Spc29 fused to the Gal4 activation domain (AD) expressed from centromeric plasmids were tested by serial dilution assays in the yeast two-hybrid system in wild-type (SLJ1644), pom152Δ (SLJ12623), and pom152Δ mps2Δ (SLJ12624). Empty binding domain and activation domain vectors were used as controls. Growth on media lacking tryptophan (Trp), leucine (Leu), and histidine (His) that also contained 25 mM 3AT (right) indicates an interaction, while growth on Trp-Leu is a plating control. Bars, 100 nm.