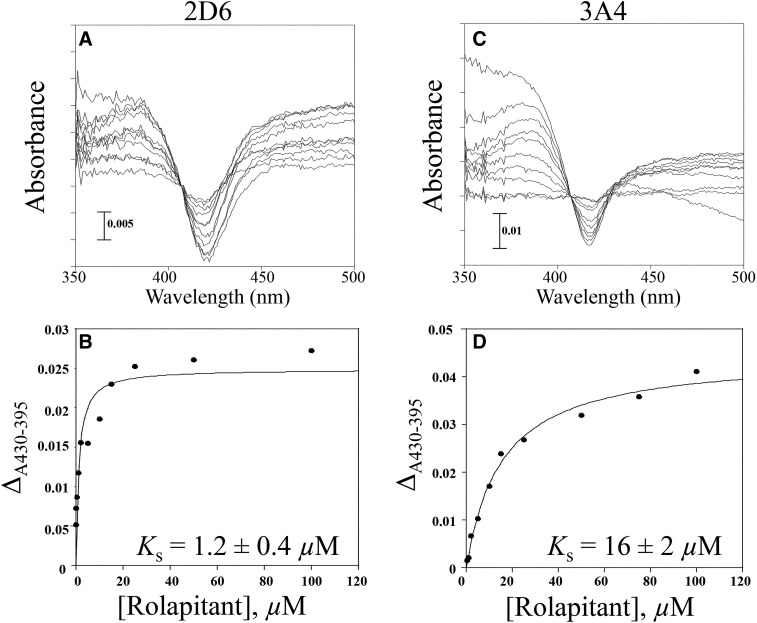
Fig. 2.
Spectral binding titration of rolapitant with CYP2D6 and CYP3A4. (A) Purified CYP2D6 (1 µM) or (B) purified CYP3A4 (2 µM) was split into two cuvettes. A baseline was taken from 350 to 500 nm. Aliquots of rolapitant were added to the sample cuvette and an equal volume of DMSO was added to the reference. Rolapitant exhibited type I spectral binding with CYP2D6 and CYP3A4, suggesting that it is a substrate for both enzymes. (C) Plot of ΔA430–395 [from (A)] vs. concentration of rolapitant. The Ks value for rolapitant with 2D6 was determined to be 1.2 ± 0.4 µM. (D) Plot of ΔA430–395 [from (B)] vs. concentration of rolapitant. The Ks value for rolapitant with 3A4 was determined to be 16 ± 2 µM.