Figure 7.

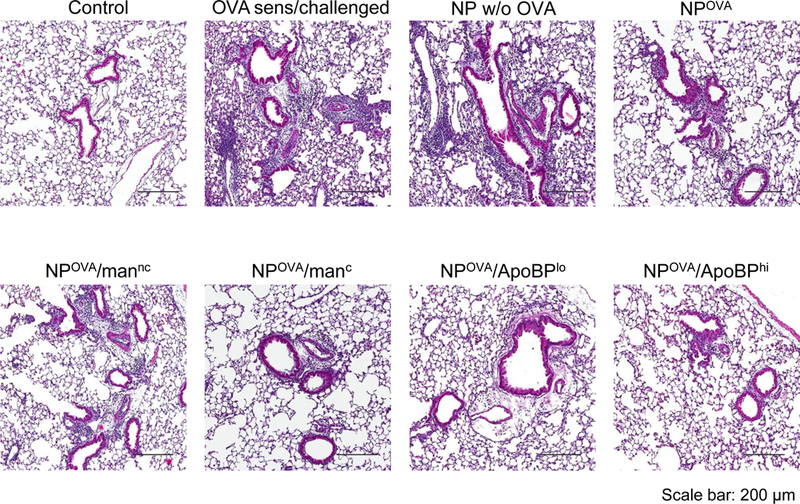
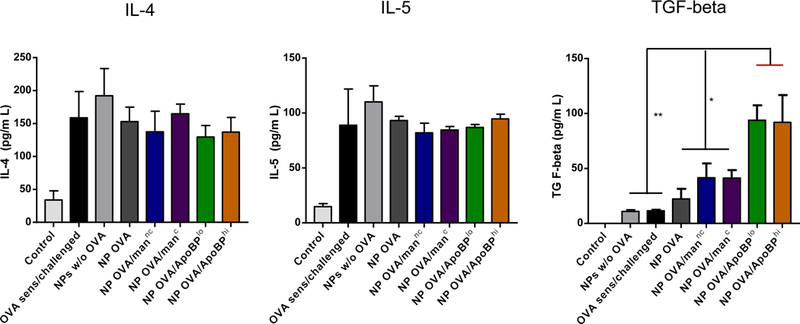
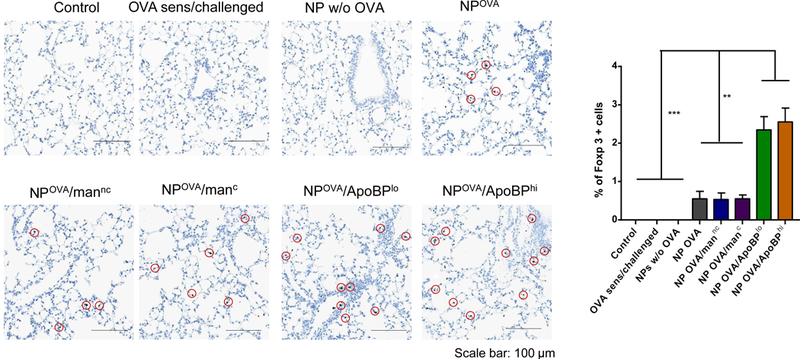
NP treatment post-sensitization alleviates allergic airway inflammation. The scheme is outlined in Fig. S5A. Briefly, 6–8-week-old C57/BL6 mice were IP sensitized with OVA (10 μg/mouse) on days 0 and 7. Subsequently, the animals received IV injection of NPOVA (with or without ligands) on two occasions days 14 and 21. The administered OVA and NP doses were similar as in Fig. 5. The post-treatment groups included: (1) a control group without any pretreatment or any sensitization or challenge; (2) no pretreatment before sensitization and challenge; (3) NPs w/o OVA; (4) NPOVA; (5) NPOVA/mannc; 6) NPOVA/manc; 7) NPOVA/ApoBPlo; 8) NPOVA/ApoBPhi. Finally, the animals received aerosolized OVA inhalation on days 35–37, as described in Fig. 5. Subsequent to animal sacrifice, BALF and lung tissue were harvested on day 40 to study the following endpoints: (A) Differential cell counts in the BALF. (B) Representative lung histology; scale bars correspond to 200 μm. (C) TH2 cytokine (IL-4 and IL-5) and TGF-β levels in BALF by ELISA. (D) IHC for Foxp3+ T-cell recruitment to the lung. Data are expressed as the mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.00 (one-way ANOVA followed by a Tukey’s test).
