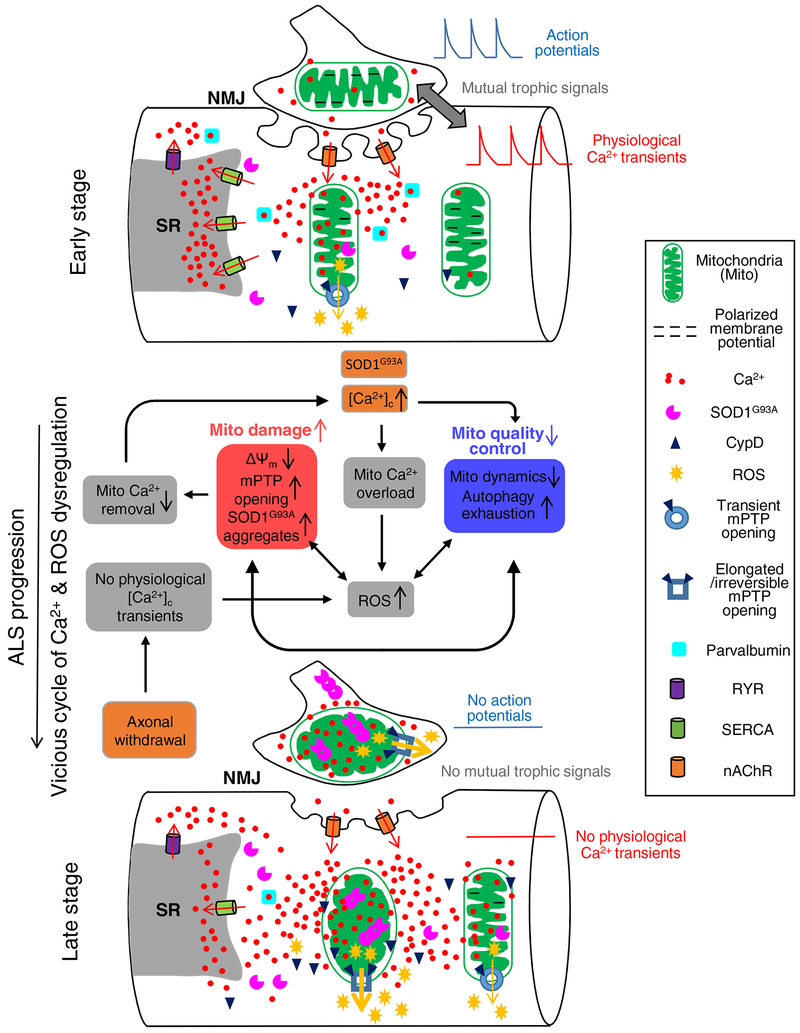
Fig 1. Proposed pathologic sequence of muscle degeneration related to mitochondrial dysfunction in the ALS mouse model.
At the early stage, the integrity of NMJ is still intact. Muscle fibers could have elevated cytosolic Ca2+ near NMJ area, potentially due to Ca2+ permeability of nAChR during repetitive muscle contraction. This elevated cytosolic Ca2+ level at NMJ could lead to mitochondria Ca2+ overload and accumulation of SOD1G93A inside mitochondria, which causes mitochondrial membrane depolarization and promotes ROS production. The observed transient opening of mPTP at this stage could be an adaptive response attempting to relieve Ca2+ overload in mitochondria. Upon the progression of ALS, accumulation of these defects would form a vicious cycle, leading to further mitochondrial damage with reduced mitochondrial Ca2+ removal and consequently enhanced cytosolic Ca2+ hyperactivity, which is exacerbated by the decreased level of SERCA1 and parvalbumin. The aggregation of SOD1G93A inside mitochondria further promotes mitochondrial damage and ROS production, inhibiting mitochondrial dynamics and exhausting autophagy capacity. The axonal withdrawal event also promotes excessive ROS production, which is accompanied by prolonged CypD-related mPTP opening. These changes lead to major consequences on abnormal energy metabolism and muscle cell death.