Fig. 2. hsBCL9CT-24 inhibits tumor growth and Wnt pathway activity in multiple CRC mouse models.
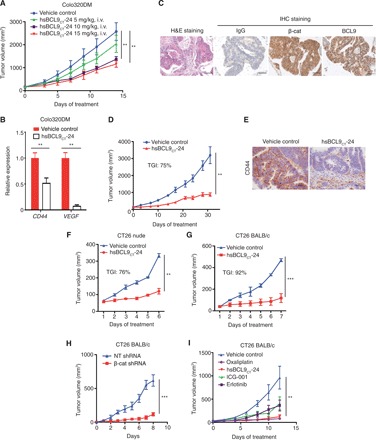
(A) Dose-escalation study of hsBCL9CT-24 treatment in the Colo320DM xenograft model. Four cohorts of female BALB/c nude mice (n = 4 per cohort) were administered vehicle control or hsBCL9CT-24 (5, 10, or 15 mg/kg) via i.v. injection, QD over 14 days. Tumor sizes are displayed as means ± SEM (**P < 0.01). (B) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) measurement of CD44 and VEGF in the Colo320DM tumors following hsBCL9CT-24 treatment (**P < 0.01). (C) Representative images of immunohistochemistry (IHC) staining for β-cat and BCL9 in a CRC patient-derived tumor tissue. H&E, hematoxylin and eosin. (D) Tumor samples in (C) were inoculated in NOD/SCID mice and treated with vehicle control or hsBCL9CT-24 at 15 mg/kg via i.p. injection, QD for 31 days (n = 8 per cohort, **P < 0.01). (E) Representative images of IHC staining for CD44 expression in the tumors from (D). Scale bar, 100 μm. (F) CT26 cells were inoculated in BALB/c nude mice before treatment with vehicle control or hsBCL9CT-24 (n = 6 per cohort) at 25 mg/kg via i.p. injection, QD for 5 days (**P < 0.01). (G) CT26 cells were inoculated in BALB/c mice before treatment with vehicle control or hsBCL9CT-24 at 25 mg/kg via i.p. injection (n = 6 per cohort), QD for 6 days (***P < 0.001). (H) CT26 cells transduced with nontargeting (NT) shRNA or β-cat shRNA were inoculated in BALB/c mice (n = 8 per cohort, ***P < 0.001); day 0 started from 10 days after tumor inoculation. (I) CT26 cells were inoculated in BALB/c mice and treated with either vehicle control, hsBCL9CT-24, or ICG-001 at 25 mg/kg via i.p. injection, erlotinib (25 mg/kg) via oral QD, or oxaliplatin (5 mg/kg) via i.p. and QD over 12 days (n = 4 per cohort, **P < 0.01). Results were denoted as means ± SEM for experiments performed in triplicate, and each experiment was repeated twice. Statistical significance of differences between groups was determined by two-way ANOVA for all tumor growth assays.
