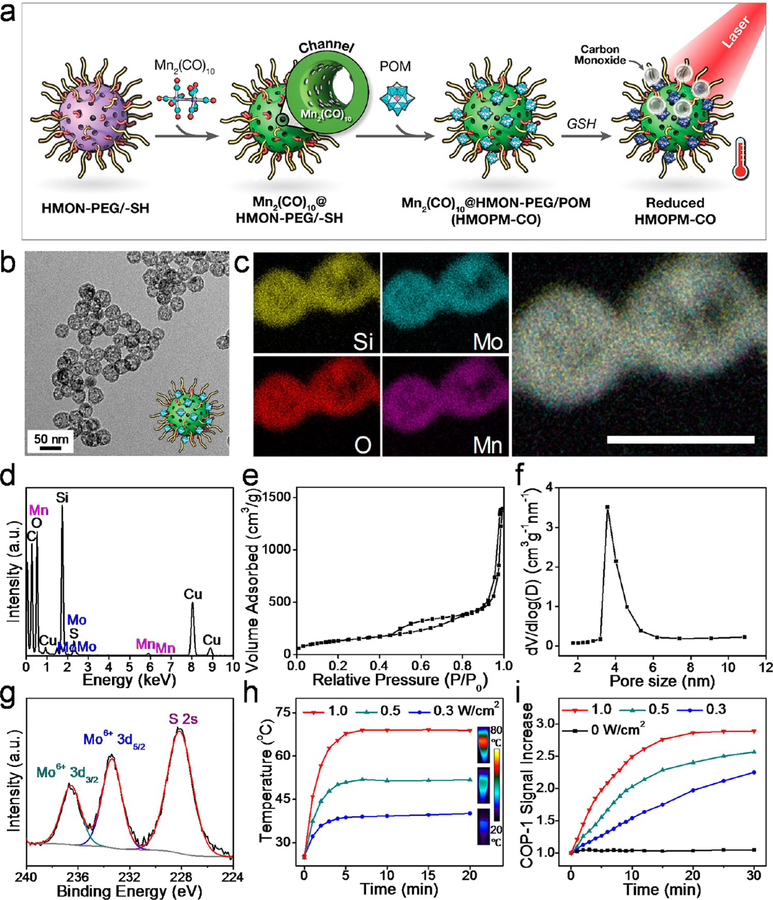
Figure 5.
Preparation and characterization of HMOPM-CO. (a) Schematic synthesis route. A typical CO releasing molecule, Mn2(CO)10, was loaded into the HMON structures via hydrophobic−hydrophobic interaction. With GSH reduction, HMOPM-CO effectively converted NIR light energy into heat, which further triggered the decomposition of Mn2(CO)10 for NIR-controlled CO release. (b) TEM image. Scale bar, 50 nm. (c) Elemental mapping. Scale bar, 50 nm. (d) EDS spectrum confirmed the successful synthesis of HMOPM-CO by presenting both Mo and Mn. (e) N2 adsorption−desorption isotherm and (f) corresponding pore-size distribution. The surface area and pore size were determined to be 491.84 m2/g and around 3.7 nm, respectively. (g) XPS spectrum of Mo 3d in HMOPM-CO. The Mo element was consistent with the valence of +6, suggesting the Mn2(CO)10 loading did not affect the subsequent POM surface modification. (h) Photothermal heating curves and images of HMOPM-CO solutions upon an 808 nm irradiation at different fluence rates. (i) The absorbance increases of COP-1 probe in response to CO generation in the irradiated HMOPM-CO aqueous solutions. The CO release amount was a function of the irradiation fluences and durations.