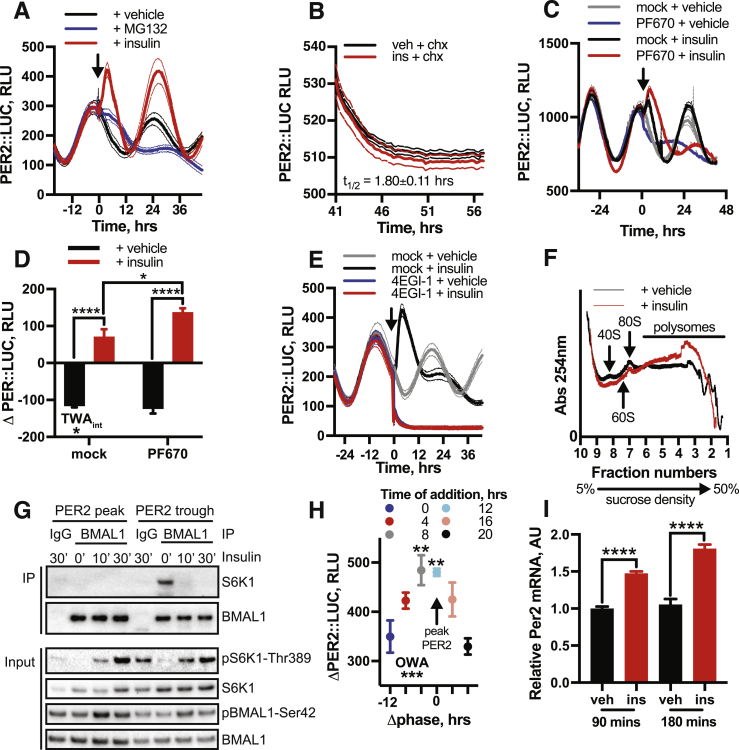
Figure S5.
Initial Induction of PER Occurs through Increased Translation of Existing mRNA, Related to Figure 4
(A) Inhibition of PER2 degradation with proteasomal inhibitor MG132 (n = 4, mean ± SEM) does not replicate the acute induction of PER2::LUC following insulin treatment (n = 4, mean ± SEM). (B) Pre-treatment with insulin does not influence PER2 degradation rate following cycloheximide, with both decay curves sharing the same half-life (n = 4, mean ± SEM, extra-sum-of-squares F test, p = 0.99), indicating that insulin does not increase PER2 levels by decreasing its rate of degradation. C, D Casein kinase 1 inhibitor PF-670462 also fails to replicate the PER2 induction following insulin (n = 4, mean ± SEM, 2-way ANOVA, Tukey’s multiple comparisons test). Consistent with insulin triggering an increase in PER2 synthesis however, we note that PF-670462 further potentiates the PER2::LUC induction following insulin treatment. (E) Inhibition of cap-dependent translation with 4EGI-1 abolishes the PER2 induction by insulin (n = 4, mean ± SEM). (F) Representative polysome profiles of absorbance at 254 nm at 60 min following treatment with vehicle or insulin (n = 3, representative). 40S, 60S and 80S peaks, and polysomes, are indicated. (G) Co-immunoprecipitation of S6K and BMAL1 at 10 and 30 min following insulin treatment shows a decrease in association of these proteins in response to insulin treatment, suggesting that BMAL1 association with translational machinery does not contribute to the acute increase in PER2 translation that follows insulin treatment. (H) Quantification of the magnitude of the PER2::LUC induction against shift in phase shows the largest induction when insulin is applied at the time when Per2 mRNA is most abundant (at 4 h before and around peak PER2::LUC), with significantly smaller inductions at the trough of PER expression, when Per2 mRNA is less abundant (n = 4, mean ± SEM, 1-way ANOVA, Dunnett’s multiple comparisons test, p-value versus the first group is shown). (I) An increase in whole-cell Per2 mRNA levels is observed at 90 and 180 min following insulin addition (n = 4, mean ± SEM, Welch’s t test). Taken together, these data suggest that, although increased PER2 transcription may contribute to some of the insulin-induced increase in PER2, increased PER translation from existing mRNA is primarily responsible for the acute increase in PER levels following insulin treatment, while the stability of PER is unaffected. All experiments were performed in PER2::LUC fibroblasts.