Figure 4: Pericentral expression of p-mTOR-S2448 is the function of GS and in turn Glutamine, downstream of the Wnt-β-catenin pathway.
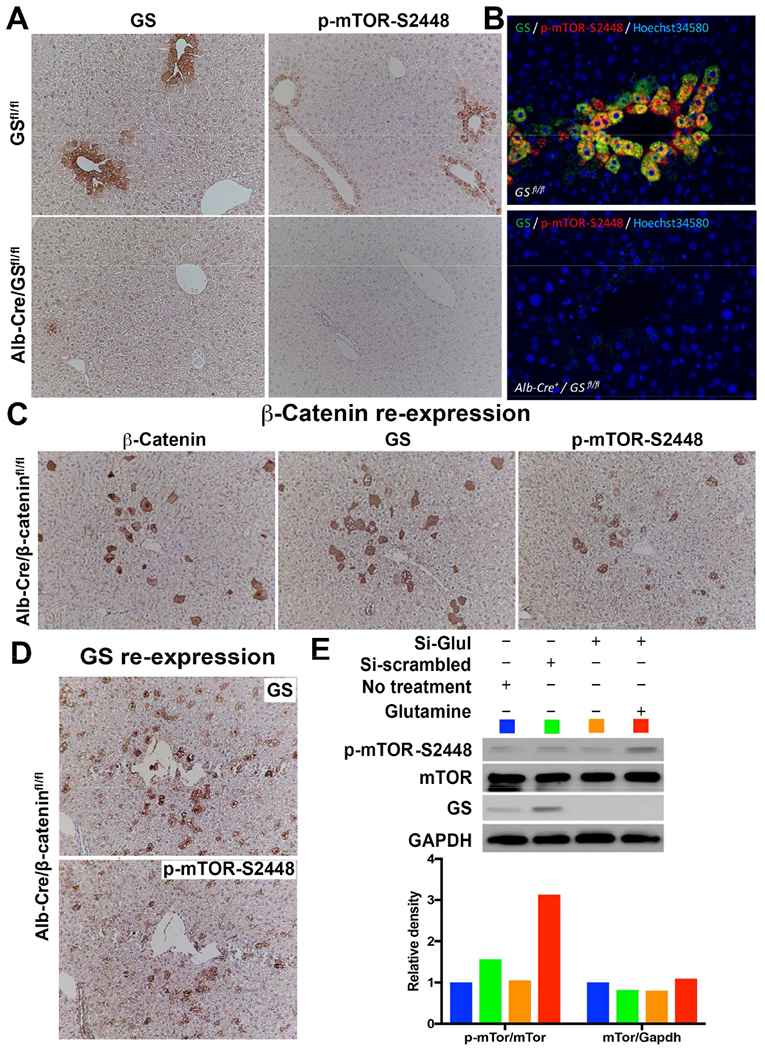
A. Conditional deletion of Glul in Alb-Cre/GSfl/fl mice leads to loss of GS and p-mTOR-S2448 in zone-3 hepatocytes (bottom), which was intact in GSfl/fl littermates (top) (100×).
B. Double immunofluorescence validates colocalization of GS and p-mTOR-S2448 in the zone-3 hepatocytes in GSfl/fl mice (top), while no staining for either was seen in the liver sections from Alb-Cre/GSfl/fl mice (bottom) (200×).
C. IHC on serial sections from livers of Alb-Cre/β-cateninfl/fl mice following forced expression of S45Y-CTNNB1 by SB-HTVI in a subset of zone-3 hepatocytes shows ectopic expression of β-catenin, GS and p-mTOR-S2448 in the same hepatocytes (100×).
D. IHC on serial sections from livers of Alb-Cre/GSfl/fl mice following forced expression of GLUL by SB-HTVI in a subset of zone-3 hepatocytes shows ectopic expression of GS and p-mTOR-S2448 in the same hepatocytes (100×).
E. GS was silenced in Hep3B cells using validated siRNA against GLUL as compared to siRNA against non-specific scrambled sequence for 24 hours, followed by supplementation with 4mM of Glutamine for another 24 hours. WB shows that GLUL siRNA successfully decreased GS as well as p-mTOR-S2448, which was restored by Glutamine supplementation. Equivalent protein loading was confirmed by GAPDH. Densitometry analysis (color-coded) on normalized sampled showed Glutamine supplementation dramatically increasing p-mTOR-S2448 after GS knockdown.
See also Figure S5.
