Abstract
Activation of the renal dopamine D1-like receptor (D1R) or angiotensin II type-2 receptor (AT2R), individually or both, simultaneously, is necessary in the normal regulation of renal sodium (Na+) transport and blood pressure. However, little is known regarding the precise mechanism of this interaction. Pharmacologic stimulation, membrane biotinylation, and cell surface immunofluorescence were used to study the effect of the D1R/AT2R interaction in human renal proximal tubule cells (RPTCs). D1R activation of GαS stimulates adenylyl cyclase and induces apical plasma membrane recruitment of AT2Rs. We now show for the first time the reciprocal reaction, AT2R stimulation with angiotensin III leads to the apical plasma membrane recruitment of the D1R. The cell-permeable second messenger analogs of cAMP (8-Br-cAMP) or cGMP (8-Br-cGMP) induce translocation of both D1R and AT2R to the plasma membrane. Inhibition of protein kinase A (PKA) with Rp-cAMPS and protein kinase G (PKG) with Rp-8-CPT-cGMPS blocks D1R and AT2R recruitment, respectively, indicating that both PKA and PKG are necessary for D1R and AT2R trafficking. Both 8-Br-cAMP and 8-Br-cGMP activate protein phosphatase 2A (PP2A) which is necessary for both plasma membrane recruitment of D1R and AT2R and the inhibition of NHE3-dependent Na+ transport. These studies provide insights into the D1R/AT2R transregulation mechanisms that play a crucial role in maintaining Na+ and ultimately blood pressure homeostasis.
Keywords: Dopamine 1 receptor, Angiotensin 2 receptor, Protein Phosphatase 2A, Renal sodium transport
Summary
This study extends previous observations about the D1R and AT2R role in regulating Na+ transport by demonstrating in human RPTCs that there is an absolute co-dependency between D1R and AT2R to inhibit Na+ transport despite their differences in second messenger linkage.
INTRODUCTION
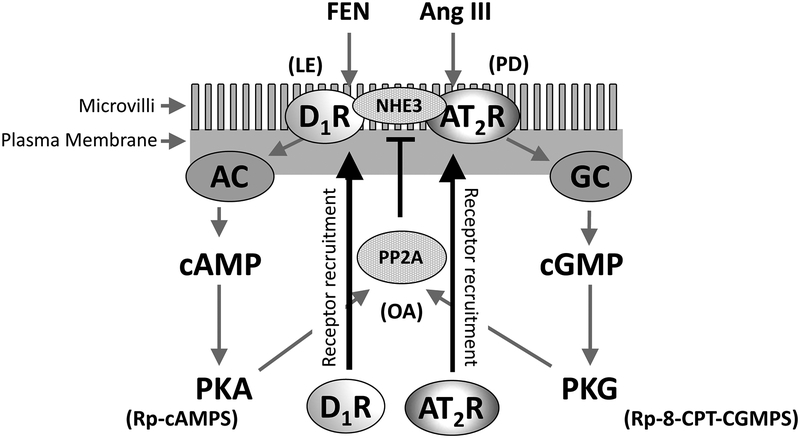
The renal dopaminergic and renin-angiotensin systems are major regulators of sodium (Na+) homeostasis1–4. Both dopamine D1-like receptors (D1R) and angiotensin II type-2 receptors (AT2R) are natriuretic receptors, as stimulation by either dopamine or angiotensin III (Ang III), respectively, inhibits renal Na+ reabsorption, resulting in natriuresis4,5. Upon agonist stimulation, D1Rs are recruited by microtubules from the cytoplasm to the apical cell surface6,7. Renal tubular plasma membrane D1Rs are further stimulated by dopamine synthesized in the renal proximal tubule cell (RPTC) that is released in a paracrine manner, increasing G protein coupling and a subsequent activation of adenylyl cyclase (AC) and protein kinase A (PKA)6,8. Renal angiotensin II (Ang II) is converted to Ang III by aminopeptidase A, which stimulates AT2Rs to decrease Na+ transport through an increase in soluble guanylyl cyclase (GC) production of cGMP which stimulates protein kinase G (PKG)9. (Figure 6).
Figure 6: Suggested Model of D1R and AT2R Recruitment to the Apical Plasma Membrane of Human RPTCs.
Fenoldopam (FEN), adenylyl cyclase (AC), protein kinase A (PKA), angiotensin III (Ang III), guanylyl cyclase (GC), protein kinase G (PKG), and okadaic acid (OA).
Previous studies have not determined whether the D1R or AT2R acts independently or collectively to inhibit renal Na+ transport. In rodents, AT2Rs are recruited to the RPTC apical plasma membrane upon D1R stimulation and the AT2R antagonist, PD-123319 (PD), blocks the D1R-mediated natriuretic response, suggesting that AT2R is necessary for D1R activity10. In human RPTCs, we and others have reported that D1R and AT2R activation increases cAMP and cGMP, respectively, leading to a decrease in Na+ transport11–14, but the interdependence of D1R and AT2R stimulation on receptor translocation to the apical plasma membrane is unknown. The goal of this study is to investigate further the relationship between the D1R and AT2R in human RPTCs. We have reported that the resensitization of the desensitized, phosphorylated D1R is dependent on its dephosphorylation by protein phosphatase 2A (PP2A)15–18. The current study tests the hypothesis that downstream activation of either D1R or AT2R converges at PP2A which is necessary for both receptors to be translocated to the apical cell surface to inhibit Na+ transport.
MATERIAL AND METHODS
The authors declare that the data that support the findings of this study are available from the corresponding author upon reasonable request.
Pharmacological agents
All pharmacological agents were purchased from Sigma-Aldrich. Fenoldopam, (FEN) is a D1R agonist; it stimulates the two D1-like receptors, D1R and D5R. 7-Methyl-6,7,8,9,14,15-hexahydro-5H-benz[d]indolo[2,3-g]azecine (LE30019) is a potent and selective D1R antagonist20. H-Arg-Val-Tyr-Ile-His-Pro-Phe-OH, Angiotensin III (Ang III) is the preferred endogenous AT2R agonist21. 1-[[4-(Dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid ditrifluoroacetate, PD-123319 (PD) is a potent and selective non-peptide antagonist to the AT2R22. 8-Bromoadenosine cyclic 3’,5’-monophosphate (8-Br-cAMP) is a cell-permeable cAMP analog. 8-Bromoguanosine cyclic 3’,5’-monophosphate (8-Br-cGMP) is a cell-permeable cGMP analog23. Rp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Rp-cAMPS) is a PKA antagonist24. 8-(4-chlorophenylthio) guanosine-3,5-cyclic monophosphorothioate, Rp-isomer triethylammonium salt (Rp-8-CPT-cGMPS) is a PKG antagonist25. 9,10-Deepithio-9,10-didehydroacanthifolicin, Okadaic Acid (OA) is a PP2A antagonist14. Ouabain (OUB) is a Na+/K+ATPase alpha inhibitor. 3,3′-(4-Methyl-1,2-phenylene)bis[N-(aminoiminomethyl)-2-methyl-2-propenamide] dihydrochloride, S3226, is a potent and selective NHE3 inhibitor26
Human RPTCs
Human kidney tissue and cell culture procedures were followed as reported previously27,28. For more details, please see the Online Supplement.
Quantification of Cell Surface Recruitment of D1R and AT2R by Flow Cytometry
RPTCs were grown in iPT-G418 media in 100 mm plates for 2 days until 60–80% confluence at 37°C in full humidity with 5% CO2. Vehicle (control) and agonists were added to cells and inhibitors were added 15 min prior to the addition of receptor agonists. Cells were stained in suspension and stained without permeabilization according to details found in the online supplement. Treated and stained cells were run on a benchtop flow cytometer with a robotic autoloader (Accuri C6Sampler, Accuri Cytometers).
Quantification of Cell Surface Recruitment of D1R and AT2R by 96-Well In-Cell Western
Procedures were followed as reported previously29. For more details, please see the Online Supplement.
Biotinylation and Western Blot Analysis
Procedures were followed as reported previously14,28. For more details, please see the Online Supplement.
PP2A Activity Assay
RPTCs at 80% confluence in 100 mm plates were incubated in serum-free DMEM-F12 media with or without agonists, washed, and lysed in phosphate-free buffer, according to kit instructions (Millipore, cat# 17–313). Briefly, 500 μg of protein were immunoprecipitated using a monoclonal antibody specific for the catalytic subunit of PP2A (clone 1D6). Phosphatase activity was measured by the release of phosphate from a phosphorylated peptide K-R-pT-I-R-R using malachite green by absorbance at 620 nm in a 96-well plate reader (BMG, Pherastar FSX) and compared to a standard curve made by diluting phosphate directly in malachite green.
NHE3-Mediated Na+ Accumulation Assay
Procedures were followed as reported previously26. For more details, please see the Online Supplement.
Specific Protocols
1. Effects of D1-Like Agonist FEN±D1R Antagonist LE300 and the Effects of Endogenous AT2R Agonist Ang III±AT2R Antagonist PD on D1R and AT2R Recruitment Measured by Flow Cytometry.
Human RPTCs were treated as follows: (1) VEH (N=12); normal media, (2) FEN (N=6); 1 μmol/L for 30 min, (3) FEN+LE300 (N=6); pretreated with LE300 (1 μmol/L) for 15 min followed by 30 min co-incubation with FEN, (4) LE300 (N=6); 30 min, (5). Ang III (N=6); 10 nmol/L for 30 min, (6) Ang III+PD (N=6); pretreated with PD (1 μmol/L) for 15 min followed by 30 min co-incubation with Ang III, and (7) PD (N=6); 30 min.
2. Dose Response Curves for 8-Br-cAMP and 8-Br-cGMP to Measure Maximal D1R and AT2R Recruitment by Flow Cytometry.
Human RPTCs were treated as follows: 8-Br-cAMP and 8-Br-cGMP for 30 min (N=6 per dose): 10 mmol/L, 3 mmol/L, 1 mmol/L, 0.3 mmol/L, 0.1 mmol/L, 0.03 mmol/L, and 0.01 mmol/L.
3. Effects of 8-Br-cAMP and 8-Br-cGMP on D1R and AT2R Recruitment Measured by Flow Cytometry and Western Blot Analysis.
Human RPTCs were treated as follows: (1) VEH (N=15); normal media, (2) 8-Br-cAMP (N=15); 1 mmol/L for 30 min, (3) 8-Br-cGMP (N=15); 1 mmol/L for 30 min, and (4) 8-Br-cAMP+8-Br-cGMP (N=15); 30 min. In a separate set of experiments (N=3 for each condition), RPTCs were treated with either 8-Br-cAMP or 8-Br-cGMP to measure both D1R and AT2R recruitment by Western blot analysis.
4. Effects of 8-Br-cGMP±PKG Inhibitor Rp-8-CPT-cGMPS and the Effects of 8-Br-cAMP±PKA Inhibitor Rp-cAMPS on D1R and AT2R Recruitment Measured by Flow Cytometry.
Human RPTCs were treated as follows: (1) VEH (N=12), normal media, (2) 8-Br-cGMP (N=6); 1 mmol/L for 30 min, (3) 8-Br-cGMP+Rp-8-CPT-cGMPS (N=6); pretreated with Rp-8-CPT-cGMPS (100 μmol/L) for 15 min followed by 30 min co-incubation with 8-Br-cGMP, (4) Rp-8-CPT-cGMPS (N=6); 30 min, (5) 8-Br-cAMP (N=6); 1 mmol/L for 30 min, (6) 8-Br-cAMP+Rp-cAMPS (N=6); pretreated with Rp-cAMPS (100 μmol/L) for 15 min followed by 30 min co-incubation with 8-Br-cAMP, and (7) Rp-cAMPS (N=6); 30 min.
5. Effects of 8-Br-cAMP and 8-Br-cGMP on PP2A Activity.
Studies followed the same protocol stated in Protocol 3 and N=3 for each condition.
6. Effects of PP2A Inhibition with OA on 8-Br-cAMP- and 8-Br-cGMP-Induced D1R and AT2R Recruitment Measured by Flow Cytometry.
Human RPTCs were treated as follows: (1) VEH (N=6); normal media, (2) 8-Br-cAMP (N=6); 1 mmol/L for 30 min, (3) 8-Br-cGMP (N=6); 1 mmol/L for 30 min, (4) 8-Br-cAMP+OA (N=6); pretreated with OA (10 nmol/L) for 15 min followed by 30 min co-incubation with 8-Br-cAMP, (5) 8-Br-cGMP+OA (N=6); pretreated with OA for 15 min followed by 30 min co-incubation with 8-Br-cGMP, and (6) OA (N=6); 30 min.
7. Effects of PP2A Inhibition with OA on Na+ Transport.
Human RPTCs were treated as follows: (1) VEH (N=6); normal media, (2) 8-Br-cAMP (N=6); 1 mmol/L for 30 min, (3) 8-Br-cGMP (N=6); 1 mmol/L for 30 min, (4) 8-Br-cAMP+8-Br-cGMP (N=6); both for 30 min, (5) 8-Br-cAMP+OA (N=6); pretreated with OA (10 nmol/L) for 15 min followed by 30 min co-incubation with 8-Br-cAMP, (6) 8-Br-cGMP+OA (N=6); pretreated with OA for 15 min followed by 30 min co-incubation with 8-Br-cGMP, (7) 8-Br-cAMP+8-Br-cGMP+OA (N=6); pretreated with OA for 15 min followed by the co-incubation with 8-Br-cAMP+8-Br-cGMP, and (8) OA (N=6); 30 min.
Statistical Analysis
The data are presented as mean ± SEM. One-way ANOVA, followed by Tukey’s test was used to compare ≥3 groups. A two-tailed Student’s t-test was used to compare 2 groups. P<0.05 was considered statistically significant.
RESULTS
Flow Cytometry Analysis of the Effects of Exogenously Added FEN and Ang III on D1R and AT2R Recruitment.
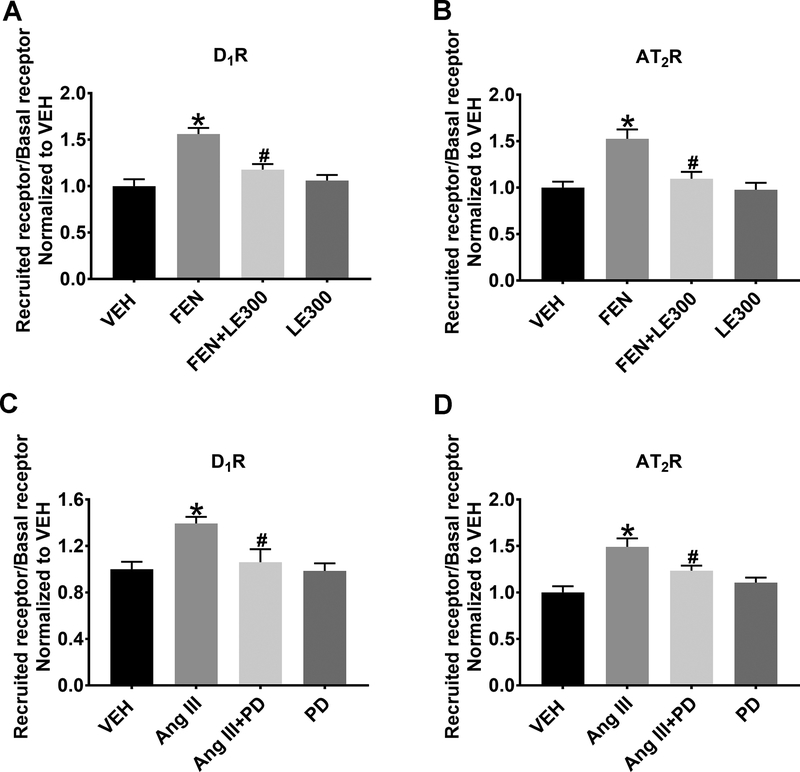
Figure 1A. RPTCs stimulated with FEN (1 μmol/L, 30 min) increased the recruitment of the D1R to the apical plasma membrane (1.56±0.07-fold, FEN vs. VEH, P<0.05, N=6). The addition of the D1R antagonist LE300 (1 μmol/L, 30 min) + FEN significantly inhibited D1R membrane recruitment (FEN vs. FEN+LE300, P<0.05, N=6 per group), while LE300, alone, had no effect compared to VEH. Figure 1B. RPTCs stimulated with FEN also increased the recruitment of the AT2R to the apical plasma membrane (1.53±0.12-fold, FEN vs. VEH, P<0.05, N=6 per group), which was inhibited in the presence of LE300 (FEN vs. FEN+LE300, P<0.05, one-way ANOVA, N=6 per group). LE300 alone had no effect on AT2R recruitment compared to VEH. Figure 1C. RPTCs stimulated with Ang III (10 nmol/L, 30 min) increased recruitment of the D1R to the apical plasma membrane (1.39±0.06-fold, Ang III vs. VEH, P<0.05, N=6 per group). The addition of the AT2R antagonist PD (1 μmol/L, 30 min) + Ang III significantly inhibited D1R membrane recruitment (Ang III vs. AngIII+PD, P<0.05, N=6 per group), while PD alone had no effect compared to VEH. Figure 1D. RPTCs stimulated with Ang III increased the apical plasma membrane recruitment of the AT2R (1.49±0.10-fold, Ang III vs. VEH, P<0.05, N=6 per group). The addition of the AT2R antagonist PD+Ang III significantly inhibited AT2R membrane recruitment (Ang III vs. AngIII+PD, P<0.05, N=6 per group), while PD alone, had no effect compared to VEH. This is the first time AT2R stimulation with Ang III is shown to induce D1R apical plasma membrane recruitment.
Figure 1: Flow Cytometry Analysis of the Effects of Exogenous FEN and Ang III Treatment on D1R and AT2R Recruitment.
FEN (1 μmol/L, 30 min) significantly increases cell surface D1R (A) and AT2R (B) in human RPTCs. *P<0.05 vs. VEH and #P<0.05 vs. FEN. Ang III (10 nmol/L, 30 min) significantly increases cell surface D1R (C) and AT2R (D), respectively. *P<0.05 vs. VEH and #P<0.05 vs. Ang III.
Dose-Response Analysis of 8-Br-cAMP and 8-Br-cGMP
Dopamine binding to the D1R leads to AC activation and accumulation of cAMP30. Similarly, Ang III binding to the AT2R leads to GC activation and accumulation of cGMP31. Using cell permeable analogs of cAMP and cGMP, 8-Br-cAMP and 8-Br-cGMP, respectively, allows us to study downstream signaling leading to D1R and AT2R membrane trafficking. Concentration-response curves were measured for both 8-Br-cAMP and 8-Br-cGMP stimulation of D1R and AT2R apical plasma membrane recruitment, using a variant of an In-Cell Western blotting technique, where the directly labeled antibodies are added to cells without cell permeabilization. Semi-log dilutions from 10 mmol/L to 10 nmol/L for both 8-Br-cAMP and 8-Br-cGMP were added to RPTCs for 30 min and both D1R and AT2R plasma membrane recruitment were analyzed. A concentration-response curve for 8-Br-cAMP and 8-Br-cGMP showed maximal recruitment of the D1R and AT2R at 1 mmol/L for both 8-Br-cAMP Figure S1A and S1B and 8-Br-cGMP Figure S1C and S1D. For the D1R, the EC50 of 8-Br-cAMP was 296.1 μmol/L (Log EC50 was −3.529) and the EC50 of 8-Br-cGMP was 144.3 μmol/L (Log EC50 was −3.841). For the AT2R the EC50 of 8-Br-cAMP was 206.4 μmol/L (Log EC50 was −3.685) and the EC50 of 8-Br-cGMP was 165.6 μmol/L (Log EC50 was −3.781).
Flow Cytometry Analysis of 8-Br-cAMP, 8-Br-cGMP, or 8-Br-cAMP+8-Br-cGMP Stimulation on D1R and AT2R Recruitment
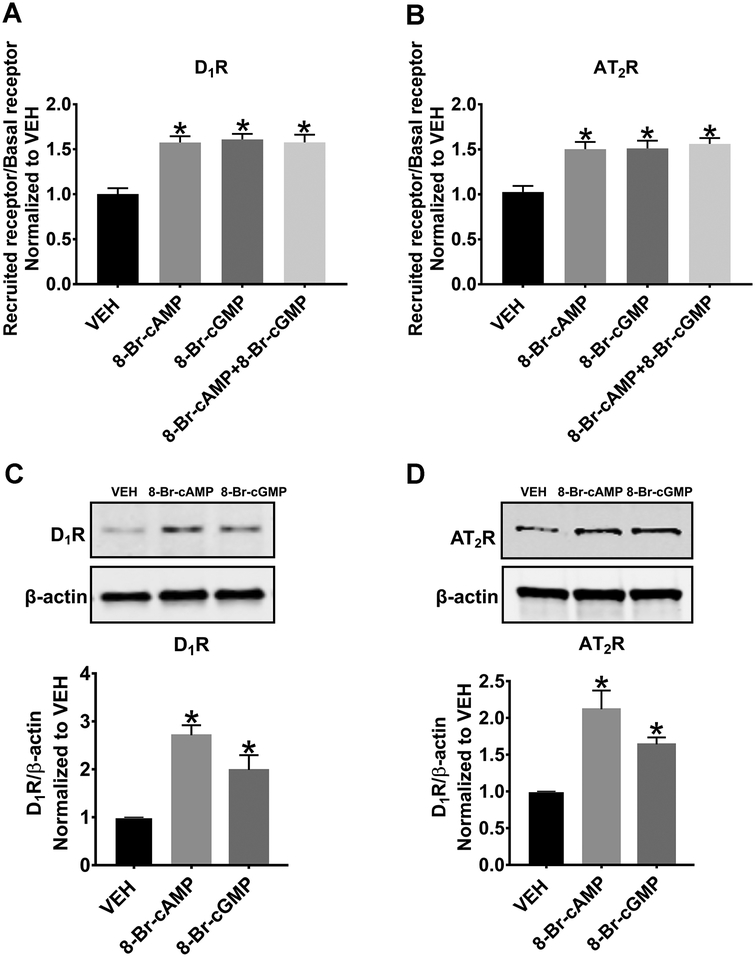
The Emax of 1 mmol/L, determined from Figure 2 for both 8-Br-cAMP and 8-Br-cGMP, measured by In-Cell Western experiments, was confirmed using flow cytometry (Figure 2A and 2B). 8-Br-cAMP, 8-Br-cGMP, or 8-Br-cAMP+8-Br-cGMP significantly increased the plasma membrane recruitment of D1R (1.50±0.08-, 1.51±0.08-, and 1.56±0.0-fold, respectively, P<0.05, N=15 per group). 8-Br-cAMP, 8-Br-cGMP, or 8-Br-cAMP+8-Br-cGMP also significantly increased the plasma membrane recruitment of AT2R (1.58±0.07-, 1.61±0.06-, and 1.58±0.08-fold respectively, P<0.05, N=15 per group). Interestingly, the simultaneous addition of both 8-Br-cAMP and 8-Br-cGMP did not cause an additional increase in apical plasma membrane recruitment, relative to either agonist alone.
Figure 2: Flow Cytometry and Western Blot Analysis of the Effects of Exogenously 8-Br-cAMP and 8-Br-cGMP Treatment on D1R and AT2R Recruitment.
8-Br-cAMP (1 mmol/L, 30 min) or 8-Br-cGMP (1 mmol/L, 30 min) significantly increases cell surface D1R (A) and AT2R (B) in human RPTCs measured by flow cytometry. *P<0.05 vs. VEH. 8-Br-cAMP or 8-Br-cGMP also significantly increases cell surface D1R (C) and AT2R (D) measured by Western blot analysis. All blots are normalized to β-actin and then to VEH. *P<0.05 vs. VEH.
Biotinylation and Western Blot Analysis of 8-Br-cAMP and 8-Br-cGMP Stimulation on D1R and AT2R Recruitment
Biotinylation and Western blot analysis were performed to verify the results generated from flow cytometry and In-Cell Western experiments. We measured D1R (Figure 2C) and AT2R (Figure 2D) recruitment when the cells were stimulated with 8-Br-cAMP and 8-Br-cGMP (both at 1 mmol/L) for 30 min. RPTCs stimulated with 8-Br-cAMP and 8-Br-cGMP induced an increase in apical plasma membrane-localized D1R expression by 2.71±0.21-fold (P<0.05) and 1.98±0.31-fold (P<0.05), N=3 per group, respectively. 8-Br-cAMP and 8-Br-cGMP also induced an increase in apical plasma membrane-localized AT2R expression by 2.11±0.42-fold (P<0.05) and 1.64±0.10-fold (P<0.05), N=3 per group respectively. These results are consistent with the flow cytometry results, indicating that the flow cytometry technique is a reliable method for measuring receptor recruitment.
Flow Cytometry Analysis of the Effects of PKG and PKA Inhibitors on Cell Surface D1R and AT2R Recruitment.
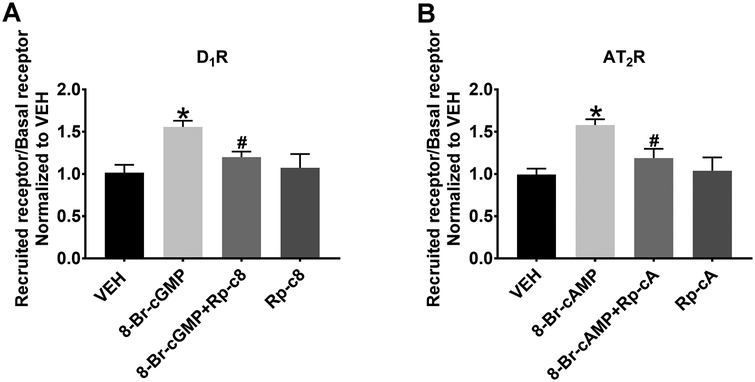
In order to determine, more precisely, the signaling downstream of cAMP and cGMP, we next examined the effect of PKA and PKG inhibition on D1R and AT2R recruitment in RPTCs. PKA and PKG are inhibited by Rp-cAMPS and Rp-8-CPT-cGMPS, respectively for 15 min prior to 8-Br-cAMP and 8-Br-cGMP stimulation. Exogenous 8-Br-cGMP (1 mmol/L, 30 min) increased the apical plasma membrane recruitment of D1R (1.56±0.05-fold vs. VEH, *P<0.05, N=6), which was inhibited in the presence of the PKG inhibitor Rp-8-CPT-cGMPs, (100 μmol/L, #P<0.05, 8-Br-cGMP+Rp-c8 vs. 8-Br-cGMP, N=6). Rp-8-CPT-cGMPS, alone, had no effect (Figure 3A). Exogenous 8-Br-cAMP (1 mmol/L, 30 min) recruited AT2Rs to the cell surface (1.58±0.04-fold vs. VEH, *P<0.05, N=6), which was inhibited in the presence of the PKA inhibitor Rp-cAMPS (100 μmol/L, #P<0.05, 8-Br-cAMP+Rp-cA vs. 8-Br-cAMP, N=6). Rp-cAMPS alone had no effect (Figure 3B). These results demonstrate that in the absence of PKA or PKG, neither D1R nor AT2R can be recruited to the cell surface, suggesting important roles for PKA and PKG in D1R and AT2R recruitment.
Figure 3. Flow Cytometry Analysis of the Effects of PKG and PKA Inhibition on D1R and AT2R Recruitment.
(A). In human RPTCs, 8-Br-cGMP (1 mmol/L, 30 min) recruits D1R to the cell surface and the PKG inhibitor Rp-8-CPT-cGMPS (Rp-c8; 100 μmol/L) blocks this response. *P<0.05 vs. VEH and #P<0.05 vs. 8-Br-cGMP. (B). 8-Br-cAMP (1 mmol/L, 30 min) recruits AT2R to the cell surface and the PKA inhibitor Rp-cAMPS (Rp-cA; 100 μmol/L) blocks this response. *P<0.05 vs. VEH and #P<0.05 vs. 8-Br-cAMP.
The Importance of PP2A in D1R and AT2R Recruitment
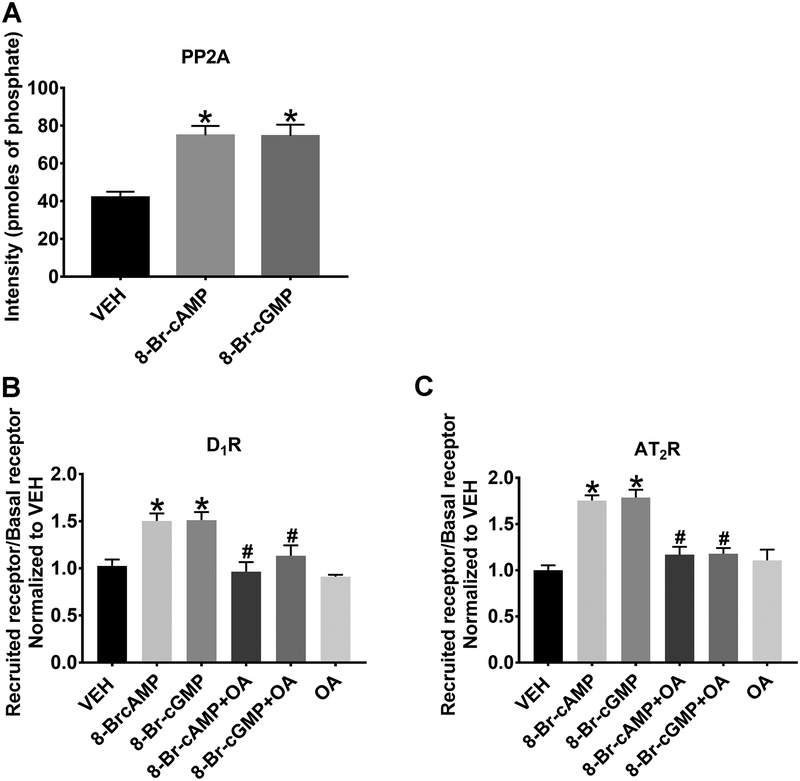
We have previously shown that the addition of the D1R agonist FEN to RPTCs leads to the activation of PP2A15–18 and AT2R cell surface recruitment, which are inhibited in the presence of OA. However, we have not tested whether cAMP or cGMP is capable of directly activating PP2A and whether OA is capable of blocking 8-Br-cAMP- and 8-Br-cGMP-dependent D1R or AT2R apical plasma membrane recruitment. Figure 4A depicts the amount of phosphate released from the phosphorylated PP2A target peptide. PP2A activity increased by 1.78±0.12-fold (*P<0.05 vs. VEH, N=3) following the addition of 8-Br-cAMP. The addition of 8-Br-cGMP also induced a 1.77±0.14-fold increase in PP2A activity (*P<0.05, N=3). Either 8-Br-cAMP or 8-Br-cGMP stimulated the recruitment of D1Rs to the cell surface (1.50±0.08-fold and 1.51±0.09-fold, respectively, *P<0.05, N=15) (Figure 4B). Either 8-Br-cAMP or 8-Br-cGMP also stimulated the recruitment of AT2Rs to the cell surface (1.76±0.05-fold and 1.79±0.08-fold, respectively, *P<0.05, N=15) (Figure 4C). Recruitment of either D1R or AT2R using cell permeable downstream second messenger agonists 8-Br-cAMP or 8-Br-cGMP was completely blocked by OA (10 nmol/L), a specific PP2A inhibitor.
Figure 4: The Importance of PP2A on D1R and AT2R Recruitment.
(A). 8-Br-cAMP (1 mmol/L, 30 min) or 8-Br-cGMP (1 mmol/L, 30 min) increases PP2A activity in human RPTCs. *P<0.05 vs VEH. 8-Br-cAMP or 8-Br-cGMP significantly increases cell surface D1R (B) and AT2R (C) recruitment that is blocked with concomitant okadaic acid (OA, a specific PP2A inhibitor; 10 nmol/L) treatment. *P<0.05 vs. VEH and #P<0.05 vs. 8-Br-cAMP or 8-Br-cGMP.
Na+ Transport–Na+ Influx Assay
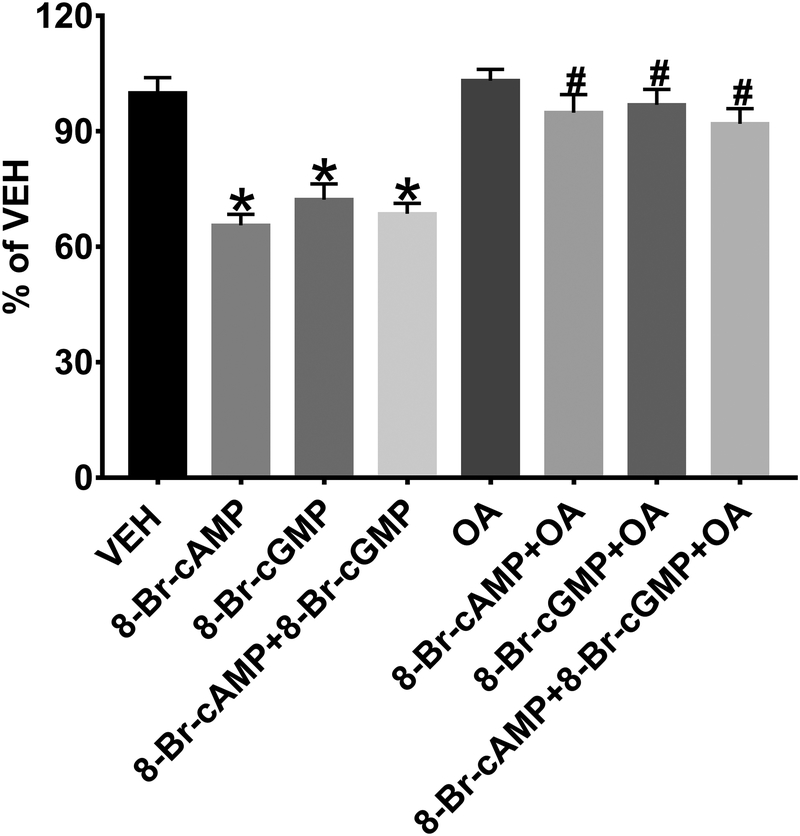
Both the D1R and the AT2R, as well as their respective endogenous agonists, dopamine and Ang III, respectively, have been implicated in inhibition of proximal tubule Na+ reabsorption that results in natriuresis. Using this cell culture model, we tested whether direct cytoplasmic stimulation with 8-Br-cAMP or 8-Br-cGMP inhibits Na+ transport and whether PP2A activity is necessary for this inhibition (Figure 5). 8-Br-cAMP (1 mmol/L, 30 min), 8-Br-cGMP (1 mmol/L, 30 min), or both 8-Br-cAMP+8-Br-cGMP significantly inhibited NHE3-mediated Na+ influx, (34.37±1.48, 27.74±1.58, 31.38±1.24 % reduction vs. VEH, respectively, *P<0.05, N=6) with no increase in the effect when combined. The PP2A inhibitor OA (10 nmol/L, 30 min) completely blocked the inhibition of NHE3-mediated Na+ influx, caused by 8-Br-cAMP, 8-Br-cGMP, or both 8-Br-cAMP+8-Br-cAMP (N=6/group).
Figure 5: Sodium (Na+) Transport – Na+ Influx Assay.
8-Br-cAMP (1 mmol/L, 30 min), 8-Br-cGMP (1 mmol/L, 30 min), or both 8-Br-cAMP+8-Br-cGMP significantly inhibits NHE3-mediated Na+ influx. *P<0.05 vs. VEH. The PP2A inhibitor okadaic acid (OA, 10 nmol/L) blocks the inhibition of NHE3-mediated Na+ influx caused by 8-Br-cAMP, 8-Br-cGMP, or 8-Br-cAMP+8-Br-cAMP. #P<0.05 vs. 8-Br-cAMP, 8-Br-cGMP, or 8-Br-cAMP+8-Br-cAMP.
Model Depicting Molecular Interactions
The model in Figure 6 summarizes our results. A slice of plasma membrane is depicted with attached microvilli. D1R and AT2R previously recruited to the apical plasma membrane appear on the membrane surface. Stimulation by D1R agonist FEN and the endogenous AT2R agonist Ang III [inhibited by the D1R and AT2R antagonists LE 300 (LE) and PD, respectively] causes an increase in D1R and AT2R recruitment to the apical plasma membrane (solid arrows) through a common serine/threonine PP2A-mediated mechanism. PP2A is stimulated via the D1R stimulation of cAMP production via AC and PKA activation, as well as AT2R stimulation of cGMP production via GC and PKG activation.
DISCUSSION
Cell surface membrane recruitment of G protein-coupled receptors from intracellular compartments is crucial to the renewal of response to autocrine and paracrine agonist stimulation. We have presented evidence that the D1R- and AT2R-mediated inhibition of RPTC Na+ transport are linked in order to assure redundancy in Na+ transport regulation. The cellular messengers associated with these receptors, PKA and PKG, are essential for D1R and AT2R recruitment through downstream activation via cAMP and cGMP.
Previous studies demonstrated that the D1R inhibition of renal Na+ transport depends on activation of the AT2R10,21, Simultaneous stimulation of D1R and AT2R induces an increase in cGMP32. D1R stimulation can also induce the recruitment of not only D1R, but also AT2R to the apical brush border membrane of human RPTCs14. However, cAMP, cGMP, or AT2R stimulated D1R translocation has not been reported. In the current study cell permeant cAMP and cGMP were able to induce both D1R and AT2R recruitment. The use of both compounds simultaneously does not synergistically or additively increase D1R and AT2R recruitment to the plasma membrane. The dose chosen for both 8-Br-cAMP and 8-Br-cGMP is the minimum dose that shows maximal translocation of these receptors and may have depleted the limited pool of internalized receptors. Alternatively the doses used could have maximally stimulated a common saturable shared pathway like PP2A. The selectivity of each cyclic nucleotide is quite high because of the relative affinities to their respective kinases, and the dose responses curves show that a dose of one tenth the chosen dose does not significantly activate recruitment of either receptor, suggesting that direct cross-activation is unlikely. The interdependence of these pathways is supported by the fact that Ang III stimulation of the AT2R is also uncoupled to cGMP in the SHR33. The D1R is also uncoupled from cAMP, in the SHR and Dahl salt-sensitive rat34,35. Future studies will focus on the role of dietary Na+ on the synthesis and release of dopamine relative to that of conversion of Ang II to Ang III.
The shared molecular mechanism how PKA or PKG activation of PP2A leads to translocation of D1R and AT2R is unknown. G Protein-Coupled Receptor Kinase 4 (GRK4) is known to phosphorylate and inactivate the D1R and may be involved in a particular inherited form of human hypertension5. β-Arrestin2 is involved with internalization and inactivation of the D1R36. We hypothesize that PP2A dephosphorylates the D1R, releasing the receptor from engagement with this well studied internalization mechanism. We have previously shown that the D1R and AT2R are found together by protein-protein interaction14, and perhaps are translocated together when released from internal pools.
Renal interstitial cGMP inhibits renal tubule Na+ reabsorption via PKG, which regulates Na+ excretion37,38. In this study, the inhibition of either PKA or PKG prevents AT2R and D1R translocation respectively to the cell surface. This novel finding further extends the receptor-cAMP/cGMP model to include PKA and PKG which are also closely linked and that both PKA and PKG need to be present in order for D1R and AT2R to be recruited to the apical plasma membranes. We also blocked PP2A activity to determine if this is where these two pathways converge and become integrated into one signaling mechanism; our previous studies demonstrated the role of PP2A in D1R signaling15–18,39. PKA has been reported to activate PP2A by phosphorylating its regulatory subunit, B56δ40. In the renal proximal tubule B56δ associates with and dephosphorylates NHE3 to facilitate the inhibitory effect of dopamine on NHE3 activity and expression41. PP2A has also been reported to be involved in the D1R-mediated recruitment of Na+/K+ATPases from cytoplasmic pools into the basolateral membrane and its subsequent inhibition in human adenocarcinoma cells39. We now report the convergence of D1R- and AT2R-mediated inhibition of luminal Na+ transport in human RPTCs at PP2A. This effect of PP2A may be a general phenomenon because we have also reported that the D2R-mediated inhibition of inflammation is also mediated by PP2A42.
Perspective:
Previous studies have not determined whether the D1R or AT2R acts independently or collectively to inhibit up to 75% of renal Na+ transport. The phenomenon of linked G protein coupled receptors has not been readily appreciated until recently. Studies by others have demonstrated elaborate interactions such as allosteric interactions between monomer receptors either as pairs, oligomers, and receptor mosaic assemblies. These assemblies can inhibit or enhance each other’s activities, or have an absolute dependence on each other to exert their downstream activities, which is the case for the D1R/AT2R interaction. Future studies will focus on determining which other proteins are involved in the Na+ transport inhibition, as we found for PP2A, and whether there is symmetric or asymmetric allosteric modulation involved in their regulation of Na+ transport.
Supplementary Material
NOVELTY AND RELEVANCE.
What is new?
We have demonstrated that there is an absolute co-dependency between D1R and AT2R to inhibit Na+ transport despite their differences in second messenger linkage. This paper is the first to show that direct stimulation of PKA and PKG can induce both D1R and AT2R recruitment to the cell surface. Inhibition of either PKA or PKG prevents both D1R and AT2R recruitment, highlighting the co-dependency of third messengers in these mechanisms. Furthermore, these two interdependent pathways converge and increase the activity of PP2A which has been shown to increase both D1R and AT2R receptor activity.
What is relevant?
This study addresses the controversy over whether the D1R or the AT2R dominates over the regulation of Na+ excretion in the human RPTC. These studies pave the way to determine why codependent systems which regulate an important aspect of electrolyte balance are located in the same renal tubule segment.
Sources of Funding:
These studies were funded by grants from the National Heart Lung and Blood Institute (NHLBI) P01-HL-074940 and R01-HL-128189 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK039308.
Footnotes
Disclosures: None
References:
- 1.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103(47):17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley SD, Coffman TM. In hypertension, the kidney rules. Curr Hypertens Rep. 2007;9(2):148–153. [DOI] [PubMed] [Google Scholar]
- 3.Armando I, Konkalmatt P, Felder RA, Jose PA. The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl Res. 2015;165 (4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen NV. Effects of dopamine on renal haemodynamics tubular function and sodium excretion in normal humans. Dan Med Bull. 1998;45(3):282–297. [PubMed] [Google Scholar]
- 5.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2(11):637–650. [DOI] [PubMed] [Google Scholar]
- 6.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci U S A. 1998;95(10):5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruse MS, Adachi S, Scott L, Holtback U, Greengard P, Aperia A, et al. Recruitment of renal dopamine 1 receptors requires an intact microtubulin network. Pflugers Arch. 2003;445(5):534–539. [DOI] [PubMed] [Google Scholar]
- 8.Holtback U, Brismar H, DiBona GF, Fu M, Greengard P, Aperia A. Receptor recruitment: a mechanism for interactions between G protein-coupled receptors. Proc Natl Acad Sci U S A. 1999;96(13):7271–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey RM, Padia SH. Role of angiotensin AT2 receptors in natriuresis: Intrarenal mechanisms and therapeutic potential. Clin Exp Pharmacol Physiol. 2013;40: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, et al. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49(1):155–161. [DOI] [PubMed] [Google Scholar]
- 11.Bertorello A, Aperia A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990;259(6 Pt 2):F924–928. [DOI] [PubMed] [Google Scholar]
- 12.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol. 2005;25(5):322–327. [DOI] [PubMed] [Google Scholar]
- 13.Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens. 2009;18(1):28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gildea JJ, Wang X, Shah N, Tran H, Spinosa M, Van Sciver R, et al. Dopamine and Angiotensin type 2 receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension. 2012;60(2):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, et al. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70(6):1072–1079. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Yu P, Asico LD, Felder RA, Jose PA. Protein phosphatase 2A B56alpha during development in the spontaneously hypertensive rat. Clin Exp Hypertens. 2004;26(3):243–254. [DOI] [PubMed] [Google Scholar]
- 17.Yu P, Asico LD, Eisner GM, Hopfer U, Felder RA, Jose PA. Renal protein phosphatase 2A activity and spontaneous hypertension in rats. Hypertension. 2000;36(6):1053–1058. [DOI] [PubMed] [Google Scholar]
- 18.Yu CQ, Yin LQ, Tu ZT, Liu DW, Luo WP. The regulatory role of dopamine receptor D1 on PP2A via SUMO-1 modification. Eur Rev Med Pharmacol Sci. 2017;21(14):3270–3276. [PubMed] [Google Scholar]
- 19.Kassack MU, Höfgen B, Decker M, Eckstein N, Lehmann J. Pharmacological characterization of the benz[d]indolo[2,3-g]azecine LE300, a novel type of a nanomolar dopamine receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 2002;366(6):543–550. [DOI] [PubMed] [Google Scholar]
- 20.Gildea JJ, Shah IT, Van Sciver RE, Israel JA, Enzensperger C, McGrath HE, et al. The cooperative roles of the dopamine receptors, D1R and D5R, on the regulation of renal sodium transport. Kidney Int. 2014;86(1):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, et al. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60(2):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller J, Horácek V. Effect of PD 123319, an AT-2 antagonist, on renal function of the anesthetized dog: comparison with EXP 3174, an AT-1 blocker. Kidney Blood Press Res. 1997;20(5):297–301. [DOI] [PubMed] [Google Scholar]
- 23.Jin XH, Siragy HM, Guerrant RL, Carey RM. Compartmentalization of extracellular cGMP determines absorptive or secretory responses in the rat jejunum. J Clin Invest. 1999;103(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beheray SA, Hussain T, Lokhandwala MF. Dopamine inhibits na,h-exchanger via D1-like receptor-mediated stimulation of protein kinase a in renal proximal tubules. Clin Exp Hypertens. 2000;22(6):635–644. [DOI] [PubMed] [Google Scholar]
- 25.Butt E, Eigenthaler M, Genieser HG. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur J Pharmacol. 1994;269(2):265–268. [DOI] [PubMed] [Google Scholar]
- 26.Gildea JJ, Shah I, Weiss R, Casscells ND, McGrath HE, Zhang J, et al. HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertension. 2010;56(3):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gildea JJ, McGrath HE, Van Sciver RE, Wang DB, Felder RA. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. Methods Mol Biol. 2013;945:329–345. [DOI] [PubMed] [Google Scholar]
- 28.Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of NaKATPase in human renal proximal tubule cells. Hypertension. 2009;54(5):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gildea JJ, Xu P, Kemp BA, Carlson JM, Tran HT, Bigler Wang D, et al. Sodium bicarbonate cotransporter NBCe2 gene variants increase sodium and bicarbonate transport in human renal proximal tubule cells. PLoS One. 2018;3(4):e0189464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu P, Sun M, Villar VA, Zhang Y, Weinman EJ, Felder RA, et al. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal. 2014;26(11):2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey RM. AT2 Receptors: Potential Therapeutic Targets for Hypertension. Am J Hypertens. 2017;30(4):339–347. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki S, Siragy HM, Gildea JJ, Felder RA, Carey RM. Production and role of extracellular guanosine cyclic 3’, 5’ monophosphate in sodium uptake in human proximal tubule cells. Hypertension. 2004;43(2):286–291. [DOI] [PubMed] [Google Scholar]
- 33.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53(2):338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felder RA, Kinoshita S, Ohbu K, Mouradian MM, Sibley DR, Monsma FJ Jr., et al. Organ specificity of the dopamine1 receptor/adenylyl cyclase coupling defect in spontaneously hypertensive rats. Am J Physiol. 1993;264(4 Pt 2):R726–732. [DOI] [PubMed] [Google Scholar]
- 35.Felder RA, Kinoshita S, Sidhu A, Ohbu K, Kaskel FJ. A renal dopamine-1 receptor defect in two genetic models of hypertension. Am J Hypertens. 1990;3(6 Pt 2):96S–99S. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem. 1999; 274(16):10999–11006. [DOI] [PubMed] [Google Scholar]
- 37.Jin XH, Siragy HM, Carey RM. Renal interstitial cGMP mediates natriuresis by a direct tubule mechanism. Hypertension. 2001;38:309–316. [DOI] [PubMed] [Google Scholar]
- 38.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D1 and angiotensin AT2 receptor interaction in natriuresis. Hypertension. 2012;59:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecuona E, Garcia A, Sznajder JI. A novel role for protein phosphatase 2A in the dopaminergic regulation of Na,K-ATPase. FEBS Lett. 2000;481(3):217–220. [DOI] [PubMed] [Google Scholar]
- 40.Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104(8):2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobulescu IA, Quiñones H, Gisler SM, Di Sole F, Hu MC, Shi M, et al. Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A. Am J Physiol Renal Physiol. 2010;298(5):F1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Jiang X, Qin C, Cuevas S, Jose PA, Armando I. Dopamine D2 receptors’ effects on renal inflammation are mediated by regulation of PP2A function. Am J Physiol Renal Physiol. 2016;310(2):F128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.