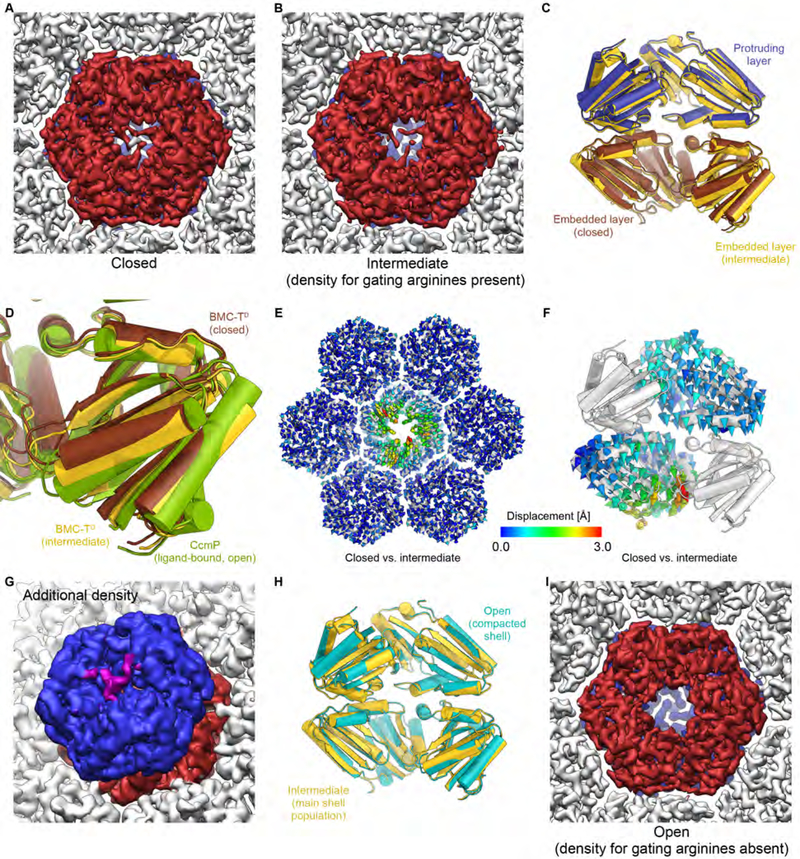
Fig. 8. Structure, conformations, and interactions of BMC-TD in the HO BMC shell.
(A) Closed conformation of the shell-embedded BMC-TD layer (dark red) in the main population of shell particles (viewed from the inside of the shell).
(B) Intermediate conformation of the inner BMC-TD ring in the main population of shell particles. Note that density for the gating arginines is still present, while the overall structure of the inner BMC-TD subunits has already undergone conformational changes (yellow state in C, D).
(C) Comparison of BMC-TD with closed inner pore (protruding trimer blue, embedded trimer brown) with BMC-TD with intermediate conformation of the inner BMC-TD ring (yellow).
(D) Detailed view of boxed region in C with additional superposition of the structure of ligand bound CcmP (green) in fully open conformation (PDB ID 5LT5) (Larsson et al., 2017).
(E) Structures of BMC-TD with closed pore and the intermediate conformation were superposed based on surrounding BMC-H, and Cα-Cα distances were plotted as color coded arrows.
(F) Same as D, but viewed from the side along the pore axis (two BMC-TD subunits are hidden to reveal the view into the channel).
(G) Weak density (purple) above the closed pore in the outer BMC-TD layer (blue) may correspond to the C-terminus of BMC-TD2. The unsharpened final map was used to generate this figure because the weak additional density is fragmented in the sharpened map.
(H) Comparison of overall conformation of BMC-TD trimers from the BMC main and compacted shell populations (intermediate and open states, respectively).
(I) Open conformation of the pore in the population of compacted shell particles (smaller apparent diameter; cyan state in H). Note the absence of density for gating arginine residues in the shell-embedded layer (dark red) and the presence of clear density for these residues in the protruding trimer in the background (blue). See also Figures S1, S4–S6, Table S1.