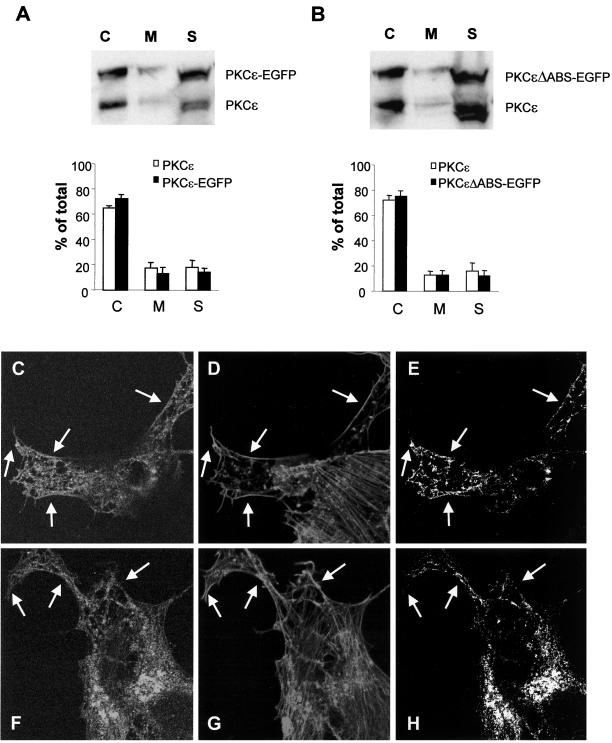
Figure 8.
Effects on the subcellular localization of PKCε by deletion of the actin-binding site. SK-N-BE(2) cells transfected with vector encoding full-length PKCε (A and C–E) and PKCε without the actin-binding site (B and F–H), both fused to EGFP. Transfected cells were subjected to subcellular fractionation (A and B) and divided into a cytosolic fraction (C), a membrane fraction (M), and a cytoskeletal fraction (S), which were analyzed with Western blotting with antibodies directed toward PKCε. Immunoblots from three individual experiments were quantified and data are presented as percentage of PKCε in each fraction out of total PKCε content. White bars represent endogenous PKCε and black bars represent EGFP-tagged PKCε variants. Cells expressing wild-type PKCε (C–E) and PKCε without actin-binding site (F-H) were fixed, and F-actin was stained with Alexa Fluor 546-conjugated phalloidin and by using confocal microscopy, a colocalization analysis was done. The images depict PKCε-EGFP (C and F), F-actin (D and G), and pixels that represent colocalization of PKCε and F-actin (E and H). Arrows highlight areas with cortical F-actin. The amount of phalloidin-positive pixels in the cortical cytoskeleton that was also EGFP-positive was 24% (wild-type PKCε) and 14% (PKCε with deleted actin-binding site) by using LaserPix software.