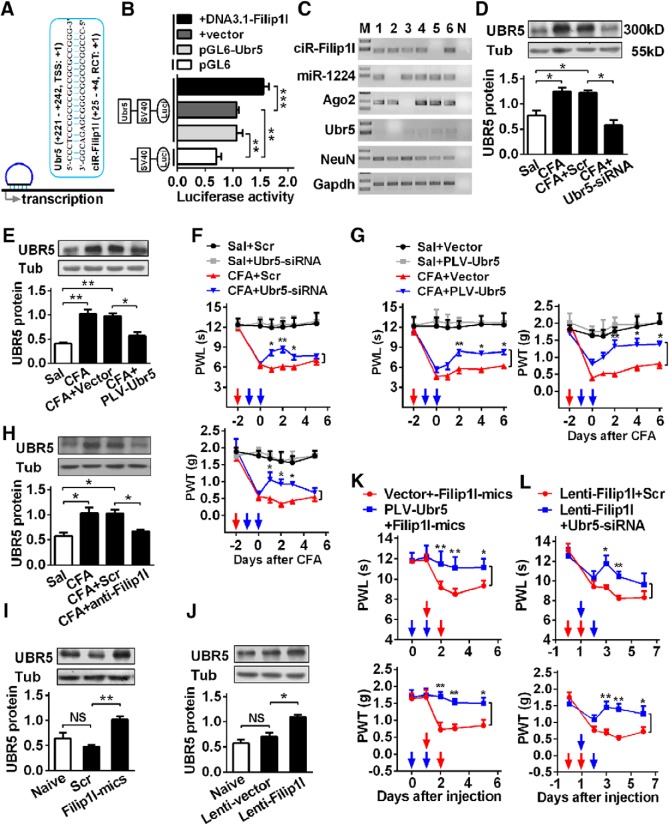
Figure 7.
circRNA-Filip1l regulates nociceptive response via positively targeting Ubr5. A, Schematic presentation of circRNA-Filip1l binding to near region of Ubr5 transcription start site (TSS). B, Validation of circRNA-Filip1l targeting Ubr5 by the use of luciferase reporter. The activities of the pGL6-Ubr5 encompassing TSS of Ubr5 region bound by circRNA-Filip1l were detected at hour 24 after cotransfection of pGL5 or pGL6-Ubr5 with DNA3.1-Filip1l by firefly luciferase reporter assays in HEK293T cells. The pGL6 plasmid (empty vector) was used as the negative control. pGL6-Ubr5, plasmid with Ubr5 region bound by circRNA-Filip1l; DNA3.1-Filip1l, plasmid of circRNA-Filip1l overexpression. Values of luciferase activities for each plasmid were normalized for transfection efficiency by cotransfection with pRL-TK plasmid. n = 4 per group. Two-way ANOVA (effect vs plasmid × treated interaction) followed by post hoc Tukey test: **p < 0.01; ***p < 0.001. C, Single-cell RT-PCR shows the coexpression of circRNA-Filip1l with miRNA-1224, Ago2, and Ubr5 in the spinal neurons of mice. No. 7 is a negative control. D, E, Knockdown of Ubr5 reversed the increase of spinal UBR5 protein 24 h after intrathecal injection of Ubr5-siRNA (D) or day 2 after 2 consecutive days of intrathecal injection of PLV-Ubr5 (E) in CFA mice. n = 5 per group. One-way ANOVA (expression vs the treated groups) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. F, Intrathecal injection of Ubr5-siRNA for 2 consecutive days alleviated the hypersensitivity to thermal or mechanical stimulus in CFA mice. n = 6 per group. Two-way ANOVA (effect vs group × time interaction) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. Red arrows indicate CFA or Sal injections. Blue arrows indicate Ubr5-siRNA or Scr injections. G, Intrathecal injection of PLV-Ubr5 for 2 consecutive days inhibited the pain sensitivity in CFA mice. n = 6 per group. Two-way ANOVA (effect vs group × time interaction) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. Red arrows indicate CFA or Sal injections. Blue arrows indicate PLV-Ubr5 or vector injections. H, Inhibiting circRNA-Filip1l via intrathecal injection of anti-Filip1l for 2 consecutive days reversed the increase of UBR5 protein in CFA mice. n = 5 per group. One-way ANOVA (expression vs the treated groups) followed by post hoc Tukey test: *p < 0.05. I, J, Upregulating circRNA-Filip1l via intrathecal injections of circRNA-Filip1l mimics (I) or Lenti-Filip1l (J) for 2 consecutive days increased the expression of UBR5 protein in naive mice. n = 5 per group. One-way ANOVA (expression vs the treated groups) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. K, Intrathecal preinjection of PLV-Ubr5 for 2 consecutive days prevented the thermal hyperalgesia and mechanical allodynia induced by circRNA-Filip1l during the development period. n = 6 per group. Two-way ANOVA (effect vs group × time interaction) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. Blue arrows indicate PLV-Ubr5 or vector injections. Red arrows indicate circRNA-Filip1l mimics or Scr injections. L, Intrathecal postinjection of Ubr5-siRNA for 2 consecutive days inhibited the pain hypersensitivity induced by Lenti-Filip1l during the development period. n = 6 per group. Two-way ANOVA (effect vs group × time interaction) followed by post hoc Tukey test: *p < 0.05; **p < 0.01. Red arrows indicate Lenti-Filip1l or Lenti-vector injections. Blue arrows indicate Ubr5-siRNA or Scr injections.