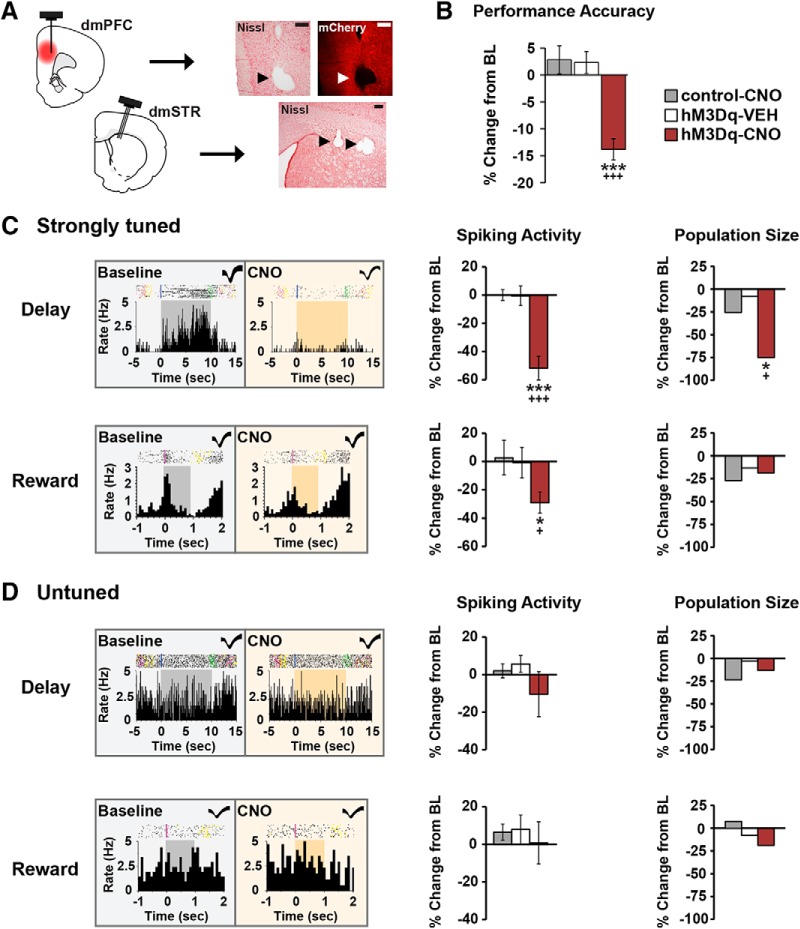
Figure 5.
Chemogenetic activation of CRF neurons in the caudal dmPFC degrades delay and reward signaling in WS dmPFC neurons. A, Left, Schematics depicting CRF-hM3Dq expression in the caudal dmPFC and electrode placements in the dmPFC and dmSTR. Right, 4× photomicrographs depicting placement of one electrode in layer V of the dmPFC and three electrodes in the dmSTR. Scale bar, 250 μm. B, In recorded animals, CNO impaired task performance in the hM3Dq group (5 animals, 7 recording sessions), but not in vehicle (4 animals, 10 sessions) or CNO-treated viral controls (3 animals, 10 recording sessions). C, Left, Exemplar rasters/PETHs demonstrating task-related activity of strongly tuned delay (top) and reward (bottom) WS neurons under baseline and CNO conditions (delay, 10 s; reward, 1 s). Middle, CNO-induced activation of dmPFC CRF neurons robustly suppressed task-related activity of strongly tuned delay (n = 24) and reward (n = 16), WS neurons relative to vehicle (delay, n = 25; reward, n = 15), and CNO-treated viral controls (delay, n = 54; reward, n = 11). Right, PFC CRF neuronal activation diminished the population size of strongly tuned delay (top), but not reward (bottom) neurons. D, Left, Exemplar rasters/PETHs of a WS neuron untuned to delay (top) and reward (bottom) under baseline and CNO conditions. CNO had no significant effects on task-related activity (middle) or population sizes (right) of these neurons in hM3Dq animals (delay, n = 55; reward, n = 65), relative to vehicle (delay, n = 40; reward, n = 59), and viral controls (delay, n = 64; reward, n = 92). *p < 0.05 versus control-CNO. +p < 0.05 versus hM3Dq-SAL. ***p < 0.001 versus control-CNO. +++p < 0.001 versus hM3Dq-SAL.