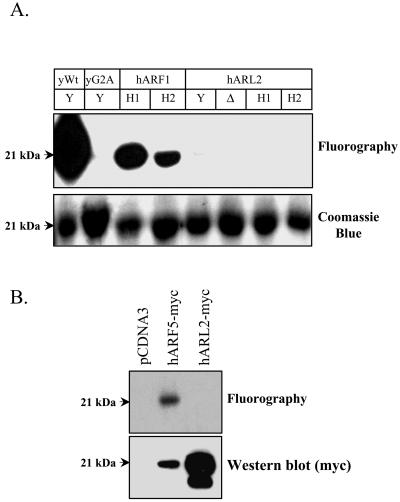
Figure 7.
ARL2 is not N-myristoylated in cultured mammalian cells or in bacteria expressing N-myristoyltransferase. (A) Covalent incorporation of [3H]myristate into human ARL2 was monitored in bacteria coexpressing S. cerevisiae (Y) NMT, human NMT1 (H1), human NMT2 (H2), or the N-terminally truncated form of human NMT1 (Δ) together with human ARL2 (hARL2), human ARF1 (hARF1), yeast ARF1 (yWT), or yeast [G2A]ARF1 (yG2A), as described under MATERIALS AND METHODS. Cell lysates were resolved in SDS gels and stained with Coomassie blue (bottom) before fluorography (top). The yeast and human ARF proteins are included as positive controls and the [G2A]ARF1 mutant as a negative control. Note the complete lack of myristate incorporated into human ARL2, despite its being expressed to similar levels as positive controls. (B) Empty vector control (pcDNA3), C-terminally tagged human ARF5 (hARF5-myc), or C-terminally tagged human ARL2 (hARL2-myc) were transiently transfected into CHO cells in the presence of [3H]myristic acid, as described under MATERIALS AND METHODS. After 16 h, expressed proteins were isolated by immunoprecipitation and resolved in SDS gels before fluorographic (top) and immunoblot detection (bottom). Note the lack of label in the ARL2 expressing cells, despite the higher levels of protein expressed relative to the ARF5 control.