Abstract
USP’s peptide reference standards content is typically determined using an HPLC assay against an external standard for which the purity was determined by a mass balance approach. To explore the use of other analytical methods, the USP Biologics Department conducted a multi-laboratory collaborative study. The study determined the inter-laboratory variability for peptide quantitation using the following methods: HPLC assay, quantitative nuclear magnetic resonance (qNMR) spectroscopy, or amino acid analysis (AAA). The three methods were compared with regard to their suitability for quantitation of the nonapeptide oxytocin. In this study, the HPLC assay method using the same peptide bulk material as the standard showed the lowest inter-lab variability. The coefficient of variation (%CV) was calculated without counting the uncertainty associated with the purity assignment of the standard with mass balance. The proton qNMR method is a direct measurement of the peptide against an internal standard, which is not difficult to perform under common laboratory conditions. Because of the simpler operation and shorter analytical time, qNMR as a primary method for peptide reference standard value assignment deserves further exploration.
Keywords: Peptide, Quantification, HPLC, NMR, Amino acid analysis (AAA)
1. INTRODUCTION
Peptide therapeutics offer the benefit of high selectivity of biologics and often can be manufactured synthetically, and are continuously gaining increased presence in the market1. To support accurate measurement of peptides in pharmaceutical development and quality control, peptide reference standards are required for calibration in liquid chromatography, mass spectrometry or other assays2. Purity determination of peptide reference standards is challenging and typically requires specialized methods.
The United States Pharmacopeia (USP) peptide reference standards (RS) have been typically packaged using bulk material. The purity of the RS was assigned by mass balance, which measures all detectable impurities and subtracts these from 100%. Because most peptides are very hygroscopic, such RS require users to weigh out the RS in a controlled humidity environment, determine water content at the time of use, and correct the water content in order to calculate the standard solution concentration.
To improve the ease of use, packaging of peptide RS has shifted to use lyophilization. This involves first dissolving the peptide bulk material in water with or without excipients, filling a pre-determined volume of the solution into ampoules or vials with high precision (%CV of fill weight is <0.50), the solvent is then removed by sublimation via the process of lyophilization. The assigned value of such prepared RS vial or ampoule is determined by assaying against a well-characterized primary standard, which is usually the same peptide bulk material whose purity has been determined by mass balance.
To improve the accuracy and precision of the assigned values of peptide RS packaged by lyophilization, a collaborative study was conducted. The study’s main objective was to evaluate and compare different analytical methods for the quantitation of peptide materials. By using the peptide RS candidates, the study focused on comparing three analytical approaches, namely quantitative nuclear magnetic resonance (qNMR), amino acid analysis (AAA), and chromatographic (HPLC) mass balance assay. qNMR utilizes its unique ability to achieve equal magnitude of response from magnetic nuclei, such as 1H, independent of chemical structure. AAA is based on quantifying stable amino acids following peptide hydrolysis. HPLC selectively quantifies an analyte of interest against a reference standard of the same analyte.
The collaborative study was conducted to address two goals:
To determine the inter-laboratory variability when measuring peptides content using one of the following methods: HPLC assay, qNMR, and AAA.
To compare the three methods above and determine which one is the most suitable for the quantitation of peptide RS, filled and lyophilized into ampoules or vials, that can be used by simply reconstitution.
In addition to USP laboratories, 11 laboratories representing industry, government, metrology, and standard-setting organizations participated in the study. Laboratory tests by all collaborators were performed between April and September 2016.
2. EXPERIMENTAL METHODS
2.1. Materials and Reagents
The materials and instruments used in this collaborative study included the following:
--USP Oxytocin Reference Standard candidate prepared by lyophilization with high-precision fill controlled by in process weight check to assess the vial-to-vial variability. The RS candidate was shipped to all collaborators from the USP, Rockville, MD, US.
--USP Oxytocin bulk material (lot B150122) was used as a standard for HPLC quantification.
--Deuterium oxide (D2O, Sigma, >99.9% D) was used as an NMR solvent by all labs.
--USP Caffeine RS Lot K0K210 (0.998 mg/mg) was used as internal standard for qNMR.
--Maleic acid (Sigma) (99.89%, certified against NIST SRM 350b benzoic acid) was used as an alternative internal standard by one lab.
-- Deuterated 2,2,3,3-d Sodium 3-trimethylsilylpropionate (TSP-d4) (Sigma, >99.9%) was used as an alternative internal standard by one lab.
--
--In-house proline, leucine, and isoleucine candidate certified reference materials with purity assigned by qNMR were used as primary calibrants and isotope labeled amino acids (Proline-13C5: CIL, Lot # PR-18738, Cat # CLM-2260-H-0.1; Leucine-13C6: CIL, Lot # PR-22233A, Cat # CLM-2262-H-0.1; and Isoleucine-13C6: CIL, Lot # PR-21540, Cat # CLM-2248-H-0.1) were used as internal standards by Lab 3.
-- L-(+)-Isoleucine, L-(+)-Leucine, L-(+)-Proline (Sigma, product #: 58879, 61819, 81709) were used as primary calibrants and isotope labeled amino acids: Isoleucine (U-13C6), Leucine (U-13C6), Proline (U-13C5, 15N) (Cambridge Isotope Laboratory, Product #: CLM-2248, CLM-2262, CNLM-436) were used as internal standard by Lab 7.
-- Amino acid standard –H (Thermoscientific, Product #:catlog: 20088) was used by Labs 2 and 9.).
In this study, the oxytocin samples were stored at −20°C, shipped on dry-ice, and the sample temperature was monitored through international cold chain shipment. Lab 1 using the HPLC method as described in section 2.8–2.9 checked the purity of oxytocin after lyophilization. Total impurities after lyophilization was 1.02% vs. 1.08% for the bulk material before lyophilization. The observed difference is within experimental limit. An accelerated stability study was conducted for the lyophilized oxytocin sample stored at −20°, 20°, 37°, and 45°C. The total impurities (%) increase rate vs. the reciprocal of temperature in Kelvin was calculated using Arrhenius equation. The correlation coefficient (r2) is 0.9933. The projected degradation rate at −20°C is 0.014%/year by Arrhenius equation. The major degradants observed are: carbimido oxytocin, acetyloxytocin, α-oxytocin dimer, and β-oxytocin dimer.
Participating Labs and Study Design:
A total of 14 labs in 10 countries took part in the study. Each lab is referred to by a code number, and Table 1 summarizes the tests done by each lab. The names of the labs are listed below alphabetically; note that there is no correlation to the order in Table 1.
Table 1:
Summary of Tests Performed by the Different Labs
| Lab No. | qNMR | AAA | HPLC |
|---|---|---|---|
| 1 | x | x | |
| 2 | x | x | x |
| 3 | x | x | |
| 4 | x | ||
| 5 | x | ||
| 6 | x | ||
| 7 | x | ||
| 8 | x | x | |
| 9 | x | ||
| 10 | x | ||
| 11 | x | ||
| 12 | x | ||
| 13 | x | ||
| 14 | x |
Note: x represents lab participation.
Aspen Oss BV, Correllistraat 10, 5344 AG Oss, Netherlands
Australian Therapeutic Goods Administration (TGA), Symonston ACT 2609, Australia
BCN Peptides S.A, Catalonia, Spain
Health Canada, Centre for Vaccine Evaluation, Ottawa ON, Canada
Ipsen Manufacturing Ireland Ltd, Dublin, Ireland
Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ), Japanese Pharmacopoeia Reference Standards Laboratory (JPRS Lab), Osaka, Japan
Swedish Medical Products Agency (Läkemedelsverket), Uppsala, Sweden
National Institute for Biological Standards and Control (NIBSC), Potters Bar, United Kingdom
National Institute of Standards and Technology (NIST), Gaithersburg, MD, United States
National Research Council of Canada (NRC), Ottawa ON, Canada
University of Nebraska, Omaha, NE, United States
US Pharmacopeial Convention (USP), Reference Standard Laboratory, Rockville, MD, United States
US Pharmacopeial Convention (USP), Reference Standard Laboratory, Hyderabad, India
US Pharmacopeial Convention (USP), Global Biologics Laboratory, Rockville, MD, United States
The procedures described in sections 2.2 through 2.9 were followed by all participants’ laboratories.
2.2. NMR Sample Preparation
Three caffeine internal standard solutions were prepared gravimetrically at (2, 3, and 4) mmol/L, respectively, in D2O. Typical caffeine internal standards were prepared by transferring 0.8 mg, 1.2 mg, or 1.5 mg of USP Caffeine RS to a 2 mL volumetric flask, dissolved and diluted to volume with D2O (0.05 wt % TSP-d4 for chemical shift referencing). Alternatively, when using maleic acid as internal standard, the solution was prepared gravimetrically at 2 mmol/L in D2O. The NMR sample solution was prepared by adding 0.5 mL of the respective internal standard solution into each vial of lyophilized oxytocin and mixing. Two vials were used at each level of internal standard solution. The oxytocin concentration was approximately 3.6 mmol/L.
2.3. NMR instrumentation
The NMR measurement was performed by transferring an aliquot (e.g., 300 µL) from each of the two vials into a suitable NMR tube (e.g. 5-mm) to combine them. The typical NMR acquisition parameters include using a 30° pulse, with a 30-second relaxation delay to ensure complete spin relaxation, and typically 64 scans. After data acquisition, Fourier transform, phasing, baseline correction, and signal integration was performed for the selected peaks of oxytocin and for the internal standard (caffeine, maleic acid, or TSP-d4). Alternatively, one lab (lab 3) measured the 1H spin-lattice relaxation time (T1) for a sample containing oxytocin and internal standard in D2O prior to qNMR analysis, using the acquisition parameters: 90° pulse with a relaxation delay of 50 seconds (approximately 7x the longest relevant T1) and 32 scans. NMR spectrometers with magnetic field strengths ranging from 300 MHz to 700 MHz were used by the collaborators.
2.4. Quantification by NMR
The determinations in this study were performed on 1D 1H NMR spectra through the proportional comparison of the peak areas integrated for both the selected signal from the internal standard and from the Oxytocin.
Where Ru is the sum of the peak areas of the signals corresponding to 4 H of tyrosine, Rs is the peak area of internal standard, ns is the number of hydrogens generating the selected signals for integration, 4 is the four hydrogens from tyrosine of oxytocin, Cs is the concentration of the internal standard solution in mg/mL, 1007.2 is the molecular weight of oxytocin in g/mol, MWs is the molecular weight of the internal standard in g/mol, V is the volume of internals standard solution pipetted into each vial (0.5 mL).
2.5. AAA Sample Preparation
The sample solution was prepared by adding a known amount of hydrolysis solution (6 to 12) mol/L HCl containing a preservative such as phenol or sodium sulfite) and an internal standard such as norleucine or deuterated amino acids to each lyophilized oxytocin sample vial. An aliquot of this solution was transferred into a hydrolysis vessel. The vessel was evacuated and sealed by melting and then heated at 110 °C to 115 °C for 12 hto 24 h. The contents of the hydrolysis vessel were cooled and dried in a vacuum. The residue was quantitatively dissolved in diluted HCl (typically 0.02 mol/L HCl). The amount of oxytocin was analyzed using a suitable amino acid analysis condition (derivatized or un-derivatized) to determine the concentrations (in nmol/mL) of the stable amino acids such as Asp, Glu, Gly, Ile, Leu, Phe, and Pro. The amount of oxytocin was calculated using the average number of nmol/mL of the amino acids found to be stable.
2.6. Quantification by AAA
For the amino acid quantitation, Lab 8 used post-column ninhydrin derivatization and Lab 9 used the pre-column OPA (ortho phthalaldehyde)/FMOC (fluorenylmethoxy chloroformate) amino acid method. Labs 3 and 7 used isotope (13C)-labeled amino acid as an internal standard to spike the sample and standard prior to hydrolysis, and the amino acids were quantified by LC-MS/MS. The peak responses selected from the stable amino acids (Asp, Glu, Gly, Ile, Leu, Phe, and Pro) are used for quantification.
Where Ru is the of the peak areas of the signals corresponding to sample, Rs is the peak area of standard, Cs is the molar concentration of the standard solution in mol/L, 1007.2 is the molecular weight of oxytocin in g/mol, V is the cumulative sample solution volume in mL.
2.7. HPLC sample preparation
The standard solutions were prepared in triplicate. The sample solutions were prepared by reconstitution. Typical sample preparation was done by pipetting 4.0 mL of water into each vial, and then mixing well by invertion. A 1:25 dilution was then made with mobile phase A (e.g.: 2.0 mL of the solution was transferred into a 50 mL vol flask, and dilute to vol with mobile phase A). Each solution was injected in duplicate. The concentrations of standard and sample solutions were 0.02 mg/mL in mobile phase A. The purity of the oxytocin standard was taken into account when generating the standard solution, the final concentration of oxytocin is 0.02 mg/mL.
2.8. HPLC instrumentation
A C18 column (250 mm × 4.6 mm, 5 μm, e.g., Waters Symmetry) was used for the gradient elution. The flow rate was 1.5 mL/min. Oxytocin was detected at 220 nm (UV), the column temperature was set to either 25 °C or 40 ° C. The standard and sample injection volumes were set at 100 µL. The mobile phases consisted of (A) 0.1 mol/L sodium dihydrogen phosphate in water and (B) a mixture of water and acetonitrile (50:50, v/v). The gradient program is shown in Table 2.
Table 2:
Gradient Program for HPLC Analysis
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 70 | 30 |
| 20 | 50 | 50 |
| 23 | 70 | 30 |
| 30 | 70 | 30 |
2.9. Quantification by HPLC
The oxytocin peak responses of the injections of sample and standard measured by HPLC are used for quantification.
Where rU is the peak response from sample solution, rT is the peak responses from standard solution, Cs is the concentration of the standard solution in mg/mL, V is the cumulative sample solution volume in mL.
3. RESULTS AND DISCUSSION
According to ICH Q23, precision may be considered at three levels: repeatability, intermediate precision, and reproducibility. Repeatability is assessed by performing multiple determinations of the same sample on the same day(s) by the same analyst and instrument in the same lab. Intermediate precision involves assessing typical variations in factors such as day, analyst, and equipment within the same lab. Reproducibility is assessed by means of an inter-laboratory trial. Reproducibility should be considered with regard to standardization of an analytical procedure. There were many published results that have evaluated the repeatability and intermediate precision of an HPLC assay, qNMR, and AAA within a single lab. In this study, the reproducibility or inter-lab precision (variability) is the key focus. The data were analyzed using SAS Proc Mixed (Windows version 9.4). Laboratory is an assumed random effect to allow for correlation of results within laboratory.
The HPLC method was an established USP compendial method. HPLC methods in the USP monographs are commonly validated following well recognized ICH guidelines. The qNMR and AAA used in this study are neither compendial nor validated. Currently, the validation of qNMR on a specific analyte such as oxytocin is not reported in the literature.
3.1. Comparison of Method Precision
3.1.1. qNMR Results
Six participating labs performed a qNMR analysis, and each tested a total of six samples. Sample numbers 1 to 3 were prepared and tested on the same day and samples 4 to 6 were prepared and tested on a different day. Samples 1 and 4 were tested using an internal standard (caffeine) with a concentration of 2 mmol/L, samples 2 and 5 were tested using an internal standard (caffeine) at 3 mmol/L, and samples 3 and 6 were tested using an internal standard (caffeine) at 4 mmol/L except for Lab 6. Lab 3 tested using caffeine [(2, 3, and 4) mmol/L] as an internal standard for Day 1 and maleic acid (2 mol/L) as an internal standard for Day 2. Lab 6 used deuterated trimethylsilylpropionic acid (TSP-d4) as the internal standard instead of caffeine or maleic acid, and Lab 6 only tested one sample at each concentration. The results (mg of oxytocin per vial) appear in Table 3.
Table 3:
Results of qNMR Tests (mg of oxytocin per vial)
| qNMR | Sample No. | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 | Lab 6 |
|---|---|---|---|---|---|---|---|
| Day 1 | 1 | 1.955 | 1.813 | 1.863 | 1.747 | 1.631 | 1.931 |
| 2 | 1.852 | 1.851 | 1.802 | 1.754 | 1.656 | 1.882 | |
| 3 | 1.800 | 1.788 | 1.793 | 1.766 | 1.632 | 1.865 | |
| Day 2 | 4 | 1.876 | 1.732 | 1.806 | 1.747 | 1.661 | |
| 5 | 1.865 | 1.823 | 1.818 | 1.755 | 1.65 | ||
| 6 | 1.834 | 1.803 | 1.831 | 1.798 | 1.664 |
Lab 1 used a 600 MHz NMR spectrometer with caffeine as the internal standard. The signals of four aromatic hydrogens at 6.8 ppm to 7.2 ppm of oxytocin (averaged) and the singlet corresponding to the aromatic hydrogen (1H) of caffeine at approximately 7.9 ppm were used for quantitation.
Lab 2 used a 500 MHz NMR spectrometer and caffeine as the internal standard. Prior to qNMR analysis, the 1H spin-lattice relaxation time (T1) was measured for a sample containing oxytocin and internal standard in D2O. The signals of four hydrogens at 6.8 ppm to 7.2 ppm of oxytocin (averaged) and the singlet corresponding to the aromatic (1H) of caffeine at approximately 7.9 ppm were used for quantitation.
Lab 3 used a 600 MHz NMR spectrometer and both caffeine and maleic acid as internal standards. The signals of four aromatic hydrogens at 6.8 ppm to 7.2 ppm of oxytocin (separately or averaged gave comparable results) and the singlet corresponding to the methyl (3H) of caffeine at 3.4 ppm to 3.5 ppm were used for quantitation for samples 1 to 3. With the alternative internal standard, maleic acid, the singlet signal at 6.22 ppm gave comparable results with better repeatability for samples 4 to 6.
Lab 4 used 300 MHz and 600 MHz NMR and caffeine as the internal standard. The signals of four hydrogens at 6.8 ppm to 7.2 ppm of oxytocin and the singlet at approximately 7.9 ppm corresponding to the aromatic (1H) of caffeine were used for quantitation. This lab reported that using either of the two aromatic signals’ responses (2H) or the sum of the two aromatic signals (4H) gave comparable assay results. Lab 4 also found that the 300 MHz (equipped with a broad band probe) and 600 MHz (equipped with a cryo probe) yielded comparable results, although there was larger measurement uncertainty at 300 MHz due to the limited resolution and sensitivity.
Lab 5 used a 500 MHz NMR spectrometer and caffeine as the internal standard. The signals of four hydrogens at 6.8 ppm to 7.2 ppm of oxytocin (averaged) and the singlet corresponding to the aromatic (1H) of caffeine at 7.9 ppm were used for quantitation.
Lab 6 used a 700 MHz NMR spectrometer and TSP-d4 as the internal standard. The signals of 11 hydrogens at 6.8 ppm to 7.2 ppm (4H), 2.8 ppm to 2.9 ppm (4H), and 2.1 ppm to 2.3 ppm (3H) of oxytocin (averaged) and the singlet at 0.0 ppm corresponding to three methyl groups (9 H) of TSP-d4 were used for quantitation.
Statistical analysis indicated that the sample mean is 1.798 mg without excluding any lab, but if Lab 6 is excluded, because of the lack of Day 2 results, it is 1.779 mg. The lab-to-lab variance (reproducibility) and the sample-to-sample variance within each lab (repeatability) are summarized in Table 4.
Table 4:
qNMR Statistical Analysisa (mg of oxytocin per vial)
| Source | Variance | Standard deviation (SD) | Relative standard deviation (%RSD) |
|---|---|---|---|
| Reproducibility (lab to lab) | 0.0072 | 0.0851 | 4.73% |
| Repeatability (sample to sample) | 0.0011 | 0.0332 | 1.85% |
The mean for all six labs was 1.798 mg with 95% confidence interval: 1.707 mg to 1.888 mg. None of the labs’ results were excluded.
3.1.2. AAA Results
Five participating labs performed the AAA test, each lab tested two samples per day over 2 days, for four samples per lab. The results from one lab (Lab 8) was invalidated due to inconsistent responses of the amino acid standard solution. The results, mg of oxytocin per vial, from the four remaining labs are presented in Table 5.
Table 5:
Results of AAA Test (mg of oxytocin per vial)
| Sample No | Lab 2 | Lab 3 | Lab 7 | Lab 9 |
|---|---|---|---|---|
| 1 | 1.775 | 1.807 | 1.740 | 1.620 |
| 2 | 1.735 | 1.833 | 1.713 | 1.644 |
| 3 | 1.807 | 1.804 | 1.718 | 1.555 |
| 4 | 1.840 | 1.808 | 1.726 | 1.603 |
Lab 2 used the hydrolysis condition as described in section 2.3. The amino acids were quantified using pre-column OPA/FMOC derivatization followed by HPLC analysis4 against an amino acid standard containing 17 amino acids derivatized concomitantly. The oxytocin content was calculated using the average content of Gly, Ile, Leu, and Pro.
Lab 3 analyzed samples as is without derivatization with LC-MS. A 6 mol/L HCl solution was used as the hydrolysis solution. The solution was heated at 110 °C for 48 h with constant stirring. There was no sample preparation or clean-up after the hydrolysis to avoid potential biases, only a 25-fold dilution was performed and used a divert valve was to minimize contamination of the mass spectrometer by chloride ion. In-house standards for Leu, Ile, and Pro were used as primary standards. 13C-derivatives of all amino acids were spiked as internal standard for samples and standards prior to hydrolysis. The analysis was carried onto a zwitterion hydrophilic interaction liquid chromatography (HILIC) column (ZIC®-HILIC, 100 mm × 2.1 mm, 3.5 µm, 100 Å) purchased from Merck SeQuant. The flow rate was 0.1 mL/min at 25°C with an injection volume of 1 µL. Mobile phases: (A) 10 mol/L ammonium acetate adjusted to pH 3.5 in H2O and (B) Acetonitrile (ACN). Mobile phases were prepared from a 200 mmol/L solution of ammonium acetate adjusted to pH 3.5 with glacial acetic acid, which was diluted 1:20 (v/v) with water and ACN, respectively. An isocratic elution was performed with 80% B during 10 min. For MS detection, a ThermoFisher TSQ Quantiva Triple Quad featuring an ESI interface was used. Source parameters in positive ionization mode were set as follows: ESI voltage of 4400 V, ion transfer tube temperature of 325 °C, vaporizer temperature of 280 °C, and collision gas pressure of 2.0 µbar. Compound-specific MS parameters, i.e., collision energy (CE) and monitoring ions (Q3), were optimized for every analyte individually by infusing a standard solution of the target compounds. Dynamic multiple reaction monitoring (MRM) was performed, setting the retention times of the analytes, with a time window of 2 min and a dwell time of 100 ms.
Lab 7 analyzed samples via vapor phase hydrolysis with monitoring of Leu, Ile, and Pro. Samples were prepared by placing them into separate glass autosampler vial inserts along with isotopically labeled amino acids used as internal standards. The samples were vacuum centrifuged to dryness. Hydrolysis was performed for 48 h at 118 °C in a sealed Teflon vessel (40 mL) containing 2 mL of 6 mol/L HCl. Each vessel also contained standards prepared for external calibration with a five-point standard curve. The quantitation method was isotope-dilution mass spectrometry coupled with HPLC. Separation was achieved by using a SIELC Primesep 100 mixed-mode (ion-exclusion and reverse phase) analytical column (2.1 mm × 250 mm, 5 μm particles, 100 Å pore size at a flow rate of 200 μL/min). An Agilent Infinity 1290 UPLC system was coupled in-line with an Agilent 6460 triple quadrupole mass spectrometer with a Jet Stream-equipped standard micro-flow source. A linear decreasing pH gradient [increasing TFA concentration from 0.05 % (v/v) to 0.45 % (v/v)] in a 20 % (v/v) organic/aqueous solvent (ACN/H2O) was used to chromatographically resolve amino acids. Column temperature was maintained at 15 °C. Mobile phases A and B consisted of 0.5 mL/L and 4.5 mL/L TFA, respectively, in 200 mL/L ACN (aqueous). Tandem mass spectrometry was performed in positive polarity mode using unit resolution for multiple reaction monitoring (MRM) of the [M+H]+ of the precursor ions. One transition was monitored for each amino acid and its isotopic internal standard. Four MRM time segments were used for MS data acquisition. The first 5 min of the chromatography gradient were sent to waste. Proline transitions were monitored from 5 min to 13 min, leucine/isoleucine from 13 min to 16.5 min. External calibration was performed via linear regression using the response and mass ratios of the standards applied to the samples.
Lab 9 used the hydrolysis condition as described in section 2.3 with 6N HCl at 110° for 21 h. The amino acids were quantified using post-column ninhydrin derivatization with the EZChrom software provided on the Hitachi L8800 to integrate chromatograms and perform regression analysis using peak heights.
Statistical analysis indicated that the sample mean is 1.733 mg. The lab-to-lab variance (reproducibility) and the sample-to-sample variance within each lab (repeatability) are summarized in Table 6.
Table 6:
AAA Statistical Analysisa (mg of oxytocin per vial)
| Source | Variance | SD | RSD |
|---|---|---|---|
| Reproducibility (lab to lab) | 0.0089 | 0.0942 | 5.44% |
| Repeatability (sample to sample) | 0.0009 | 0.0308 | 1.78% |
The mean value for the four labs was 1.73 mg with 95% confidence interval: 1.580 mg to 1.884 mg. Five labs performed AAA testing but one lab’s results were excluded due to the amino acids standard solution preparation error.
3.1.3. HPLC Assay Results
Eight participating labs performed the HPLC assay test. The design of HPLC test is a one day test by each lab, eight samples per lab. The results from one lab (Lab 10) was not included because Lab 10 was unable to obtain stable water content by Karl-Fisher, which implies insufficient humidity control during the assay standard preparation. The results in mg of oxytocin per vial from the seven remaining labs appear in Table 7.
Table 7:
Results of HPLC Assays (mg of oxytocin per vial)
| Sample No | Lab 1 | Lab 2 | Lab 8 | Lab 11 | Lab 12 | Lab 13 | Lab 14 |
|---|---|---|---|---|---|---|---|
| 1 | 1.829 | 1.851 | 1.777 | 1.801 | 1.825 | 1.720 | 1.822 |
| 2 | 1.808 | 1.861 | 1.785 | 1.822 | 1.816 | 1.740 | 1.830 |
| 3 | 1.819 | 1.852 | 1.777 | 1.815 | 1.815 | 1.706 | 1.806 |
| 4 | 1.818 | 1.910 | 1.793 | 1.798 | 1.815 | 1.734 | 1.829 |
| 5 | 1.827 | 1.861 | 1.781 | 1.819 | 1.845 | 1.726 | 1.827 |
| 6 | 1.830 | 1.857 | 1.786 | 1.823 | 1.823 | 1.737 | 1.820 |
| 7 | 1.816 | 1.843 | 1.787 | 1.819 | 1.818 | 1.721 | 1.820 |
| 8 | 1.810 | 1.859 | 1.785 | 1.822 | 1.794 | 1.747 | 1.822 |
Statistical analysis indicated that the result of 1.910 mg by Lab 2 was an outlier. With this exclusion, the sample mean was 1.806. The lab-to-lab variance (reproducibility) and the sample-to-sample variance within each lab (repeatability) are summarized in Table 8.
All Labs used the same HPLC condition, as described in sections 2.7–2.9. All collaborators also used the same USP oxytocin standard material as the HPLC assay test. The purity of the standard on an anhydrous basis was calculated by mass balance (100 – total HPLC impurities)×(100 – acetic acid – residual solvents – inorganic residue)/(100×100), in which the total HPLC impurities and acetic acid are calculated using the average of six labs’ results. The manufacturing process used for generating the oxytocin standard material ensures that residual solvents and inorganic residue contents were close to zero; this was tested and confirmed by the USP Reference Standard Lab. The water content of the standard material was measured with Karl-Fisher (KF) titration by each lab at the time of use, and the KF results were used to correct the overall purity of the standard used by each individual lab. The variability of the mass balance result could be attributed to the differences between labs in measured HPLC impurities and acetic acid content (Table 9).
Table 9:
HPLC Impurities and Acetic Acid Content (w/w%)
| Lab 1 | Lab 2 | Lab 8 | Lab 11 | Lab 12 | Lab 13 | |
|---|---|---|---|---|---|---|
| Total HPLC impurities detected in the standard | 1.08 | 1.14 | 0.93 | 0.97 | 0.80 | 0.60 |
| Acetic acid content of the standard | 7.81 | 7.83 | 7.81 | 7.52 | 7.97 | 7.90 |
3.2. Results Comparison
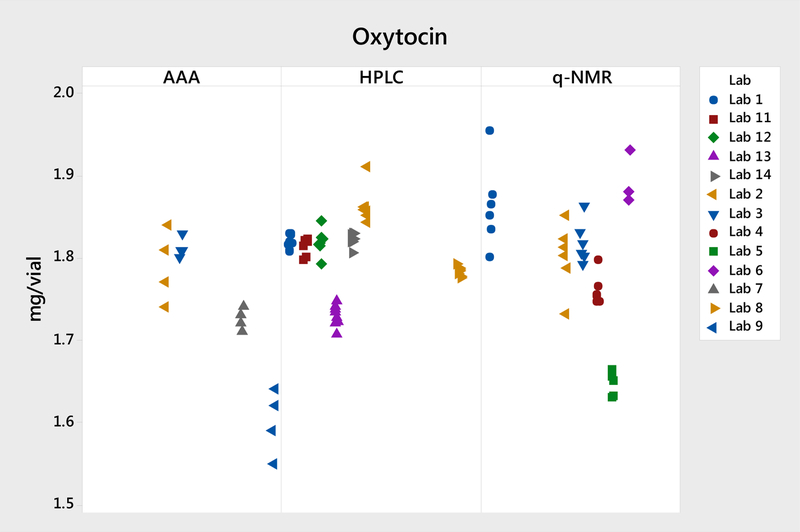
The three quantification methods yielded comparable means: 1.807 mg by HPLC assay, 1.798 mg by qNMR, and 1.73 mg by AAA. In this study, the HPLC assay yielded the least intra- and inter-lab variability. AAA appeared to have much higher intra-lab and inter-lab variability, compared with qNMR and HPLC. This higher variability is further discussed in section 3.2.2. The individual data of the three methods are illustrated in Figure 1.
Figure 1.
Plot of individual data for the peptide reference materials.
The seven labs that performed the HPLC assay used the same HPLC method, and all seven labs are highly proficient in HPLC analysis. Six labs performed the qNMR test, and five of them used a similar condition with the same internal standard—caffeine. Initially, we had anticipated better accuracy and precision for qNMR analysis because the method uses a highly pure, simple compound as the internal standard. We provided a highly characterized USP Caffeine RS to all six labs. Five of them reported results using caffeine as the internal standard and one lab reported results using in-house TSP-d4 as their internal standard. Labs 3 and 4 performed additional studies using alternative internal standards. These results are discussed in section 3.2.1.
3.2.1. Selection of Internal Standard for qNMR
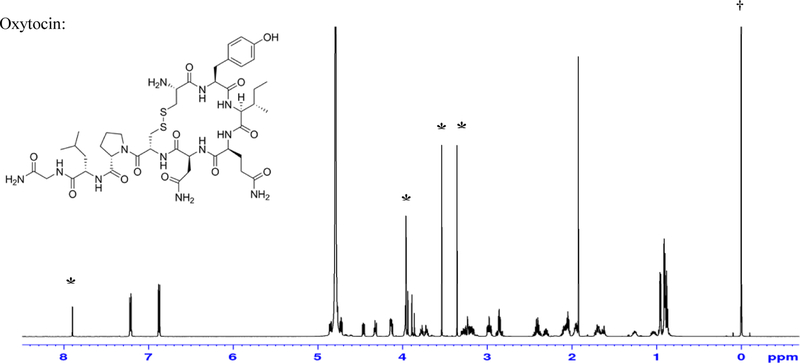
In this study, five labs used caffeine as the internal standard for quantification; the typical 1H NMR spectrum of oxytocin with caffeine is shown in Figure 2. The chemical shift (δ) of signal at 7.90 ppm from caffeine and the two signals from four hydrogen associated with tyrosine of oxytocin can be measured easily for quantification.
Figure 2.
A 600 MHz NMR spectrum of Oxytocin with internal standards caffeine (marked *) and TSP (marked †)
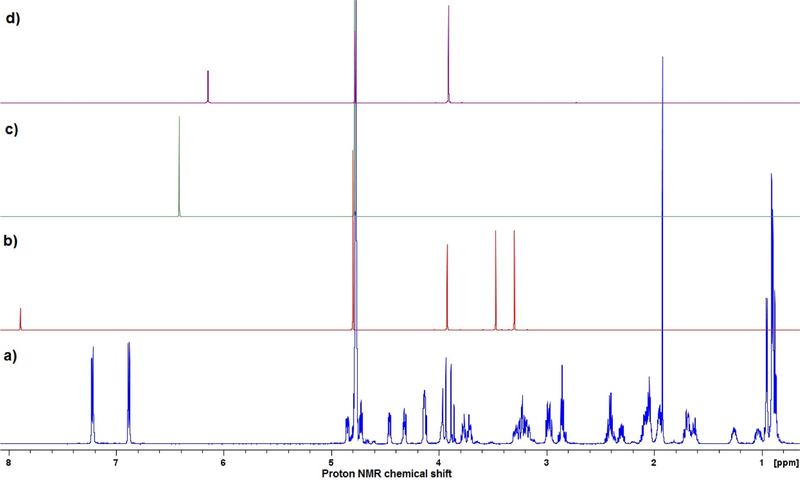
Caffeine was used as the internal standard by five laboratories. The signals associated with caffeine at approximately δ7.90 ppm did not interfere with analyte oxytocin signals at δ6.9 ppm to 7.2 ppm (see Figure 2). Reproducible NMR results thus were obtained in replicate determinations and are consistent with the assay values determined by other analytical methods. The inter-laboratory tests also confirm the suitability of this internal standard as well as the quantitation attempts, however most peptides have crowded overlapping signals in the region of 2 ppm to 4 ppm. It was reported that caffeine’s purine ring hydrogen (chemical shift δ7.90 ppm) is likely to be solvent- and/or concentration-dependent due to the possibility of exchange, and may not be optimal for quantification. However, the results obtained in the present work indicate that caffeine is a useful internal standard for quantification of oxytocin in D2O solution. Figure 3 contains the 1H NMR spectra of some typically used NMR internal standards for water-soluble compounds.
Figure 3.
Comparison of 300 MHz 1H NMR Spectra of internal standards and oxytocin. a) oxytocin, b) caffeine, c) maleic acid, and d) 3-sulfolene.
Maleic acid is readily available in pure form and has a simple 1H NMR spectrum; the singlet proton signal is unlikely to overlap with peptide signals, and may be a more suitable qNMR internal standard, for quantification of peptides, than caffeine. Another alternative would be 3-sulfolene, that shows two signals at ca. 6.2 ppm and 4.0 ppm (Ref 6). Lab 3 compared the use of maleic acid versus caffeine as an internal standard and the results are shown in Table 10. A similar amount of oxytocin was obtained with both standards, but the use of maleic acid provided better within-lab precision (repeatability). Maleic acid displays one well resolved singlet peak in the spectrum, in an often signal free part of the spectrum.
Table 10:
Oxytocin Content by qNMR
| Replicates | Oxytocin content by qNMR using caffeine as the internal standard (mg/vial) | Oxytocin content by qNMR using maleic acid as the internal standard (mg/vial) |
|---|---|---|
| 1 | 1.863 | 1.806 |
| 2 | 1.802 | 1.818 |
| 3 | 1.793 | 1.831 |
| Average | 1.819 | 1.818 |
| %RSD | 2.1% | 0.7% |
Note that selection of an appropriate internal standard is crucial for the result. Some desired characteristics of the standard are: 1) ability to provide unique and stable signals (chemical shifts), 2) high purity material is available, 3) soluble in different NMR solvents, 4) easy handling for tasks such as weighing and transfer, 5) non-volatile, 6) non-reactive, and 7) stable in the long term5.
3.2.2. Observation of Amino Acid Analysis
AAA, has been one of the earliest adopted technique for Peptide quantification measurement of the amino acids often proceeded by liquid chromatography or liquid chromatography - tandem mass spectrometry (LC-MS/MS), using carbon-13 labelled amino acids as internal standards in most cases6,7,8, 9. This approach is well-established and has been validated through international comparison exercises10. Peptide reference standard value assignment has typically been performed by amino acid analysis following peptide hydrolysis [JM3–6]. Several amino acids such as leucine, proline, valine, and phenylalanine are stable under the acid hydrolysis conditions and can be used as proxies for quantitation of the parent peptide6. AAA is widely used for peptide and protein quantitation, this study only had four labs that performed the AAA test, which is not enough to draw a conclusion about using AAA for peptide quantitation. The survey result from this study reveals that the accuracy and precision are highly dependent on the quantitation methods. The LC-MS method used by labs 3 and 7 gave low intra-lab variability, but this method used 13C isotope-labeled amino acids as an internal standard, which is not an established approach by pharmaceutical industry labs. The routine pre- or post-column derivatization methods used by Labs 2 and 9 have variability that is too high to be a top choice for peptide quantitation.
4. CONCLUSIONS
In conclusion, the study described above demonstrates the use and suitability of three distinct analytical methods for peptide quantification: HPLC, qNMR, and AAA. The merits of these approaches and the significance of the results achieved have been evaluated statistically. In this study, the traditional HPLC assay method using the same peptide bulk material as the standard had the lowest inter-lab variability.
qNMR spectroscopy has been employed for value-assignment of peptide reference standards11,12. qNMR for peptide quantitation has also been evaluated through international comparison exercises13, and relative to amino acid analysis offers the advantage of measuring peptides directly without the added complexity and potential errors associated with acid hydrolysis. qNMR is easy to perform under common laboratory conditions. Its accuracy and precision can be enhanced by selecting appropriate signals from the peptide or selecting an internal standard that has non-exchangeable and non-overlapping signals in D2O. The scope of qNMR analysis can be also applied easily to peptide reference standards packaged by lyophilization.
The common peptide impurities are diastereomers, deletion/insertion sequence, hydrolysis, oxidation, and N-, O-acetylation related14. NMR is generally not suitable for quantification of these types of product-related impurities because they usually show similar 1D 1H NMR spectra, thus making peak overlap likely. HPLC, especially LC-MS, is an easier separation tool for peptide product-related impurities. On the other hand, non-product-related impurities such as solvents can often be detected and quantitated by NMR. Statistical approaches would be required in those situations. In this study, the values assigned by NMR and AAA were not corrected for peptide related impurities, which in theory could contribute a bias comparing with the results by HPLC. The current study provides a promise for compendial adoption of the qNMR methodology. However, the roadmap to approach the validation of qNMR could be further developed. Because qNMR has the advantages of simpler operation and shorter analytical time, it deserves further exploration as a primary method for peptide assay15. For inclusion as a compendial analytical procedure, NMR would need to be introduced as more readily in pharmaceutical quality control (QC) laboratories.
Table 8:
HPLC Statistical Analysisa (mg of oxytocin per vial)
| Source | Variance | SD | RSD |
|---|---|---|---|
| Reproducibility (lab to lab) | 0.00168 | 0.0410 | 2.3% |
| Repeatability (sample to sample) | 0.00015 | 0.0123 | 0.7% |
The mean value for the six labs was 1.807 mg with 95% confidence interval: 1.769 mg to 1.845 mg.
Acknowledgment:
The authors express grateful thanks to Ms. Christina Chase, Dr. Christopher Jones, Dr. Diane McCarthy, Dr. Edmond Biba, and Dr. Yang Liu, for their helpful comments in this endeavor.
Footnotes
Note: Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
REFERENCES
- 1.Fosgerau K, Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov Today 2015;20:122–128. [DOI] [PubMed] [Google Scholar]
- 2.Vitzthum F, Siest G, Bunk DM, Preckel T, Wenz C, Hoerth P et al. Metrological sharp shooting for plasma proteins and peptides: The need for reference materials for accurate measurements in clinical proteomics and in vitro diagnostics to generate reliable results. Proteomics Clin Appl 2007;1(9):1016–35. [DOI] [PubMed] [Google Scholar]
- 3.ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1), Current Step 4 version
- 4.Long William, Automated Amino Acid Analysis Using an Agilent Poroshell HPH-C18 Column. Agilent HPLC column application note
- 5.Rundlöf Torgny, Mathiasson Marie, Bekiroglub Somer, Hakkarainen Birgit, Bowden Tim, Arvidsson Torbjörn. Survey and qualification of internal standards for quantification by 1H NMR spectroscopy. Journal of Pharmaceutical and Biomedical Analysis (JPBA) 2010; 52:645–651. [DOI] [PubMed] [Google Scholar]
- 6.Munoz A, Kral R, Schimmel H. Quantification of protein calibrants by amino acid analysis using isotope dilution mass spectrometry. Anal Biochem 2011;408(1):124–31. [DOI] [PubMed] [Google Scholar]
- 7.Kinumi T, Goto M, Eyama S, Kato M, Kasama T, Takatsu A. Development of SI-traceable C-peptide certified reference material NMIJ CRM 6901-a using isotope-dilution mass spectrometry-based amino acid analyses. Anal Bioanal Chem 2012;404(1):13–21. [DOI] [PubMed] [Google Scholar]
- 8.Bunk DM, Lowenthal MS. Isotope dilution liquid chromatography-tandem mass spectrometry for quantitative amino acid analysis. In: Alterman MA, Hunziker P, editors. Methods Mol Biol 2012. p. 29–38. [DOI] [PubMed]
- 9.Josephs RD, Stoppacher N, Daireaux A, Choteau T, Lippa KA, Phinney KW et al. State-of-the-art and trends for the SI traceable value assignment of the purity of peptides using the model compound angiotensin I. Trends Anal Chem 2018;101:108–19. [Google Scholar]
- 10.Josephs RD, Li M, Song D, Westwood S, Stoppacher N, Daireaux A et al. Key comparison study on peptide purity—synthetic human C-peptide. Metrologia 2017;54(1A):08007. [Google Scholar]
- 11.Huang T, Zhang W, Dai X, Zhang X, Quan C, Li H et al. Precise measurement for the purity of amino acid and peptide using quantitative nuclear magnetic resonance. Talanta 2014;125:94–101. [DOI] [PubMed] [Google Scholar]
- 12.Melanson JE, Thibeault M-P, Stocks BB, Leek DM, McRae G, Meija J. Purity assignment for peptide certified reference materials by combining the qNMR and LC-MS/MS amino acid analysis results: Application to angiotensin II. Anal Bioanal Chem 2018, 410 (26) : 6719–6731 [DOI] [PubMed] [Google Scholar]
- 13.Josephs RD, Li M, Song D, Daireaux A, Choteau T, Stoppacher N et al. Pilot study on peptide purity—synthetic human C-peptide. Metrologia 2017; 54 (1A):08011. [Google Scholar]
- 14.Gregg Brian, Verlander Michael, Rode Harold, Eggen Ivo, Swietlow Aleksander, Szajek Anita Y.. Control Strategies for Synthetic Therapeutic Peptide APIs. Part I: Analytical Consideration. PharmTech 38(3); 2014. [Google Scholar]
- 15.Pauli Guido F., Gödecke Tanja, Jaki Birgit U., Lankin David C.. Quantitative 1H NMR: Development and Potential of an Analytical Method: An Update. J. Nat. Prod, 2012, 75 (4): 834–851 [DOI] [PMC free article] [PubMed] [Google Scholar]