Abstract
Background
This is an update of the review on "Lidocaine for pain relief in burn injured patients" first published in Issue 3, 2007, and first updated in 2012. Pain is a major issue for people with many different types of wounds, in particular those people with burn injuries. Prompt, aggressive use of opioid analgesics such as morphine has been suggested as critical to avert the cycle of pain and anxiety, but adverse effects are encountered. It has been proposed that newer agents such as lidocaine could be effective in reducing pain and alleviating the escalating opioid dosage requirements in people with burn injury.
Objectives
To assess the safety and effectiveness of intravenous lidocaine as a means of pain relief versus no therapy, placebo, other drugs, or a combination of these therapies in people with burn injury.
Search methods
For this third update, we searched the Cochrane Central Register of Controlled Trials (Issue 11, 2013), and Ovid MEDLINE, MEDLINE in Process and Ovid EMBASE (up to December 2013).
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs), published and unpublished, which assessed the efficacy of intravenous lidocaine in varying doses as a single‐agent therapy with no therapy, placebo, other analgesics (such as opioids), lidocaine plus another drug, or a combination of these therapies as a means of pain relief in people with burn injury.
Data collection and analysis
Two review authors independently abstracted data and assessed the risk of bias of the studies identified.
Main results
In this 2014 update, we found no new studies. The one small randomised double‐blind placebo‐controlled cross‐over trial found in 2012, which included only 45 participants and compared intravenous lidocaine against placebo as a means of pain relief in people with burns still remains central to this review. We assessed this study as being at a high risk of bias due to its small size (fewer than 50 participants per treatment arm). Subjective pain ratings, as measured by the verbal rating scale, increased during procedures for both treatment arms; however, the increase was less in the lidocaine treatment group. There were no significant clinical or statistical differences regarding the effects of lidocaine and placebo on opioid requests and consumption, anxiety or level of satisfaction during a wound care procedure, but the small included study provided insufficient data to draw any conclusions.
Authors' conclusions
As current clinical evidence is based on only one RCT as well as case series and reports, intravenous lidocaine must be considered a pharmacological agent under investigation in burns care, the effectiveness of which is yet to be determined with further well‐designed and conducted clinical trials.
Plain language summary
Lidocaine for pain relief in people with burns
Background
Burns are very common and sometimes fatal, and the pain associated with such injury is one of the most difficult types to relieve. The use of high‐dose opioid medications like morphine is common, but side effects are encountered. Alternative agents such as lidocaine, an anaesthetic, have been proposed. This is an update of the review of the same name first published in 2007.
Study characteristics
We searched scientific databases for studies assessing the pain‐relieving effects of intravenous (given into the blood stream through a vein) lidocaine in adults with a burn injury. We included studies comparing lidocaine with no treatment, placebo (a pretend treatment), other analgesics (pain killers), or a combination of these. We wanted to look at (for example) severity of pain, time to requiring more medication, rescue analgesia (where extra pain relief is needed in addition to that planned) and side effects. The evidence is current to December 2013.
Key results
We found one small clinical trial, involving only 45 participants, which showed a benefit from intravenous lidocaine for pain relief in people with burns. The trial did not show a difference in opioid use, participant anxiety or level of participant satisfaction with the use of intravenous lidocaine.
Quality of the evidence
The small included study provided insufficient data to draw any conclusions.
Background
Description of the condition
This is an update of the review on "Lidocaine for pain relief in burn injured patients" first published in 2007 (Wasiak 2007). Burn injuries are a particularly emotive injury with which pain is frequently associated. Burn pain continues to be an ongoing issue of investigation and concern (Dauber 2002; Richardson 2009). Inflammatory reactions pursuant to injured tissue or nerves may result in the overproduction of inflammatory mediators, which may result in allodynia and primary hyperalgesia in injured areas and secondary hyperalgesia in surrounding tissue (Meyer 1981; Richardson 2009). Repetitive painful stimuli can cause neuroplastic adaptations throughout the central nervous system whereby pain afferent sensory impulses undergo facilitation and amplification to a given stimulus, contributing to the generation and maintenance of chronic pain (Richardson 2009). Pain from burn injuries has been associated with several mental health conditions such as depression, anxiety, post‐traumatic stress disorder and suicide (Edwards 2007; Summer 2007).
Opioid analgesics, and in particular intravenous opiates, have long been the mainstay of analgesia for procedure‐related burn pain. However, use of high‐dose opiates can be associated with both short‐term adverse effects, including respiratory depression and constipation, as well as long‐term consequences, such as the development of tolerance and reward‐based behaviour to opiate medication.
Description of the intervention
Lidocaine (also named lignocaine) is a local anaesthetic agent of the amide type that has been used for local anaesthesia and systemically as an anti‐arrhythmic drug. Intravenous lidocaine has been proposed as a means to alleviate the debilitating effects of various types of pain and is currently used in the management of many acute, chronic and neuropathic pain conditions (Edwards 1999; Ferrante 1996; Hand 2000; Koppert 2004; McLeane 2001). The most common timing of use of analgesia tends to be during debridement. Systemic lidocaine has also been reported to be effective in treating burn injury in numerous case series, case studies and experimental studies (Cassuto 2003; Holthusen 2000; Jönsson 1991).
How the intervention might work
Lidocaine reversibly binds to subunits of voltage‐gated sodium channels in the nerve membrane, consequently inhibiting nerve conduction. The depression of conduction in afferent nerves secondary to lidocaine administration may act to modify pain in people with burns, and avoid the adverse effects of high‐dose opiates during procedures associated with burn management likely to elicit repetitive painful stimuli.
Why it is important to do this review
Several clinical trials have attempted to use non‐opioid therapies to treat procedural pain with mixed degrees of success (Finn 2004; Harandi 2004; Konstantatos 2009; Zor 2010). Although many burns units employ several different therapies, there is still no widely accepted alternative or uniform adjunctive treatment to intravenous opioids for the management of procedural burn pain supported by meta‐analysis of randomised controlled trials (RCTs). Given the importance of pain control in acute burn injuries, further evidence should be sought with respect to non‐opioid analgesic agents, particularly in the context of procedural pain control. As systemic lidocaine is safe and effective for the management of some neuropathic conditions that have been refractory to standard interventions, we anticipated that we could establish that systemic lidocaine may help reduce the pain of burns.
Objectives
To assess the safety and effectiveness of intravenous lidocaine as a means of pain relief versus no therapy, placebo, other drugs or a combination of these in people with burn injury.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and controlled clinical trials, published and unpublished, that assessed the analgesic efficacy of intravenous lidocaine in people with burn injury.
Types of participants
Adults (aged over 18 years) with any burn injury who required lidocaine as a means of pain relief. We expected to include studies regardless of the method of burn injury. However, we excluded people with burn injury requiring pain relief measures for multiple treatment regimens such as skin‐grafting procedures.
Types of interventions
Intravenous lidocaine of varying doses as a single‐agent therapy compared with no therapy, placebo, other analgesics (such as opioids), lidocaine plus another drug, or a combination of these therapies regardless of the duration of the treatment.
Types of outcome measures
Primary outcomes
Pain measured by a visual analogue scale (VAS) or verbal rating scales (VRS), a numerical rating scale, or other validated assessment tool.
Categorical rating of pain intensity in circumstances in which numerical ratings may be problematic.
Time to re‐medication.
Requirements for rescue analgesia.
Secondary outcomes
We did not included studies that reported on secondary outcomes alone such as adverse effects, measures of satisfaction, physical functioning without reference to pain, emotional functioning without reference to pain, participant preference and assessment of quality of life.
Search methods for identification of studies
We ran the original search for this review in March 2007 and undertook a subsequent update in 2012. For this third update in 2014, we searched:
the Cochrane Central Register of Controlled Trials (Issue 11, 2013) (Appendix 1);
MEDLINE and MEDLINE in Process (Ovid) (to December 2013) (Appendix 2);
EMBASE (Ovid) (to December 2013) (Appendix 3).
In MEDLINE, we combined the search strategy with the optimum trial search strategy described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no language restrictions.
Data collection and analysis
Selection of studies
In the original review and with the second update, two review authors (JW and HC) reviewed titles and abstracts to identify potentially relevant trials using the selection criteria. For the 2014 update, the review authors performed a similar screening process with the help of an additional review author (HT). We did not review studies that clearly did not meet the inclusion criteria. Two review authors independently retrieved, scanned and reviewed all potentially relevant studies in full text. In all instances, we resolved differences of opinion by discussion.
Data extraction and management
Three review authors (JW, PM and SMcG) independently extracted data from the eligible studies using standardised forms developed for this review. Data extracted included: study characteristics, participant demographics, intervention and comparison details, outcome measures and results. We contacted primary authors to request missing information. In all instances, we resolved differences of opinion by discussion among the review authors.
Assessment of risk of bias in included studies
We assessed the risk of bias for each study according to the recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The 'Risk of bias' tool incorporates assessment of randomisation (sequence generation and allocation concealment), blinding (of participants, treatment providers and outcome assessors), completeness of outcome data, selection of outcomes reported and other sources of bias. We resolved discrepancies in ratings by discussion.
For this update, we assessed the included study according to size (low risk of bias: 200 or more participants per treatment arm; unclear risk of bias: 50 to 199 participants per treatment arm; high risk of bias: fewer than 50 participants per treatment arm).
Measures of treatment effect
If possible, we expressed dichotomous data as risk ratios (RR) and 95% confidence intervals (CIs). We expressed continuous data as mean difference (MD) and 95% CIs. We planned all analyses to be made on intention‐to‐treat (ITT) results. However, none of the studies used such analyses.
Assessment of heterogeneity
If possible, we tested statistical heterogeneity using the Chi2 test with significance at P value < 0.10 and a quantification of the degree of heterogeneity using the I2 statistic, and planned to carry out further exploration using subgroup analyses.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were pre‐specified.
Results
Description of studies
Results of the search
In the original review in 2007, we identified 25 references. Independent scrutiny of the titles and abstracts identified five potentially relevant studies. Of the five potentially relevant studies, we excluded four studies because they did not address primary outcome measures (Holthusen 2000; Mattsson 2000), or used alternative study designs such as case reports (Cassuto 2003), or case series (Jönsson 1991). In the 2012 update, we identified 406 further references, of which one additional study, undertaken by the review authors (JW, AS and HC) met the inclusion criteria (Wasiak 2011); one study was identified for exclusion (Koppert 2004). In this latest update, we examined an additional 18 studies but none were suitable for inclusion. See Figure 1.
1.
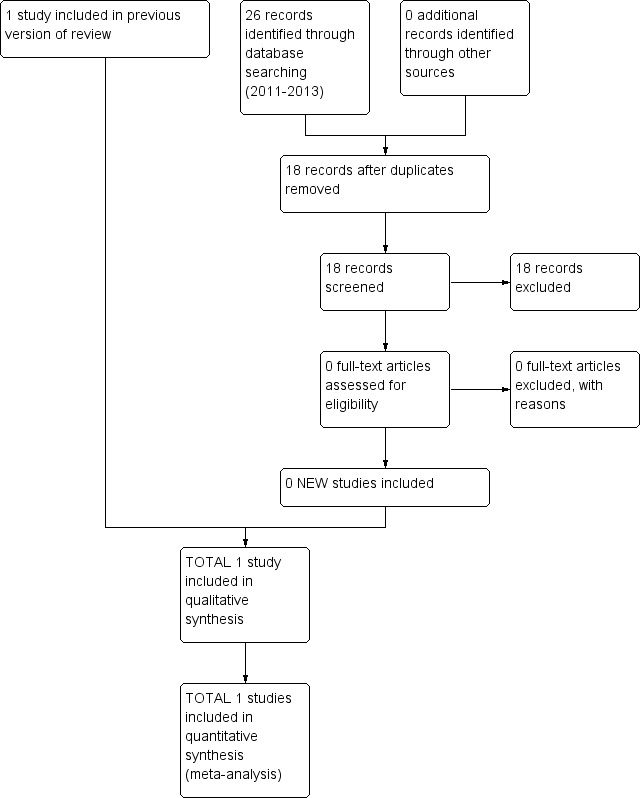
Study flow diagram.
Included studies
We included one small cross‐over RCT that we found in 2012 (Wasiak 2011). In Wasiak 2011, 47 participants were initially recruited although only 45 participants had burn injuries (mean per cent total body surface area (TBSA) 12.96%, range 3% to 55%). Participants were undergoing wound care procedures (i.e. dressing changes or debridement, or both) over two consecutive days following split skin‐graft surgery. They were randomised to receive lidocaine 1.5 mg/kg/body weight followed by two boluses of 0.5 mg/kg at five‐minute intervals followed by a continuous infusion or control (0.9% sodium chloride) administered at an equivalent volume, dose and rate to that of lidocaine. The primary outcomes measured were change in VRS scores measured before, during and after the procedure; time to rescue analgesia; opioid requests and consumption using patient‐controlled analgesia (PCA); and overall participant anxiety and level of satisfaction. Subjective pain ratings as measured by the VRS increased during procedures for both treatment arms; however, the increase was less for the lidocaine treatment arm. Table 1 presents the results for the individual study.
1. Results of individual study.
| Study ID | Dose of lidocaine | Comparator | Number of participants | Withdrawals | Efficacy | Adverse events (intervention) | Adverse events (placebo) |
| Wasiak 2011 | Initial bolus dose of lidocaine 1.5 mg/kg/body weight followed by 2 boluses of 0.5 mg/kg/body weight at 5‐minute intervals and an infusion run at 2 mg/minute throughout duration of dressing | 0.9% NaCl (normal saline) at equivalent dose, rate and volume as intervention | 45 | The authors reported no loss to follow‐up of participants for primary outcome measures | Verbal rating scale scores lower for lidocaine compared with placebo. No difference between groups in time to rescue analgesia, opioid requests and consumption using patient‐controlled analgesia, and overall participant anxiety and level of satisfaction | Pre‐dressing: twitchiness 1/45 and nausea/vomiting 8/45 Dressing: twitchiness 1/45 and nausea/vomiting 5/45 Post‐dressing: nausea/vomiting 8/45 |
Pre‐dressing: nausea/vomiting 2/45 Dressing: nausea/vomiting 4/45 and severe pain 1/45 Post‐dressing: nausea/vomiting 4/45 |
Excluded studies
In this update, we added no excluded studies. For previous excluded studies, see the Characteristics of excluded studies table.
Risk of bias in included studies
We based risk of bias assessment on the methods outlined in The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011) (Figure 2, Figure 3). The authors who were not involved in the RCT (SMcG, SD) commented on the individual study in the 'Risk of bias' section of the Characteristics of included studies table. Using the definitions provided by Higgins (Higgins 2011), the study by Wasiak 2011 described adequate allocation concealment and blinding of participants, staff and study researchers. For this update, we also identified a high risk of bias according to the size of the study (as it only had 45 participants), and updated the summary tables accordingly. Although Wasiak 2011 indicated an ITT analysis, the study had two participants lost to follow‐up as one dressing was changed in the outpatient setting and the other stopped due to uncontrolled pain.
2.
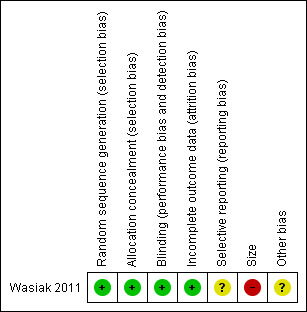
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Wasiak 2011 reported that VRS scores were significantly lower for lidocaine (0.34) compared with placebo (0.70) when measured before, during and after the procedure (difference 0.36, 95% CI 0.17 to 0.55; P value < 0.001). Rescue analgesia was required during six wound care procedures, all involving the removal of staples. During the six procedures, all participants had required intravenous ketamine during dressing procedures prior to rescue analgesia; four participants during the lidocaine arm, one during the placebo arm and one during both. Comparison of opioid demand and consumption showed no significant clinical or statistical differences between lidocaine and placebo arms. Measures of overall anxiety and satisfaction levels showed no appreciable difference between the groups. There were no significant clinical or statistical differences between the lidocaine and placebo arms with regards to anxiety scores prior to or after the wound care procedures.
Discussion
This review aimed to assess the safety and effectiveness of intravenous lidocaine as a means of pain relief versus no therapy, placebo, other drugs or a combination of these therapies in people with to burn injury.
Intravenous lidocaine has been a well‐documented treatment for other clinical conditions such as cardiac arrhythmias and neuropathic pain. In the setting of burns pain, there has been growing evidence in the form of case reports or case series to suggest that lidocaine can improve the analgesic efficacy, alleviate the deleterious effect of opioid administration and minimise the necessity of escalating opioid dosages in people with thermal injury although we excluded these from our original search (Cassuto 2003; Edwards 1999; Jönsson 1991).
Several mechanisms have been proposed for this action, namely that systemic lidocaine can depress conduction in afferent nerves, inhibit dorsal horn neural transmission and modify the cerebral perception of pain (Abelson 2002; Attal 2000). In addition, it has been postulated that burn injury is likely to trigger the release of inflammatory agents such as histamine, serotonin and prostaglandins, which in turn could trigger nociceptive impulses, making lidocaine's potent anti‐inflammatory properties integral to the suppression of pain (Edwards 1999).
We included only one small study involving the addition of lidocaine infusions to PCA morphine as compared with placebo involving 45 randomised people with burn injury (Wasiak 2011). Outcomes included pain intensity as measured by VRS, time to rescue analgesia, opioid requests and consumption, and overall anxiety and level of satisfaction. There were statistically significant differences in VRS score, which was lower in people treated with lidocaine as compared with placebo; however, there were no statistically significant differences with respect to other primary outcomes. Of importance, lidocaine administration did not result in a significantly lower demand by participants for opioids. The study was conducted in a burns centre in Melbourne, Australia where the authors of the trial acknowledge that the standard opioid‐based pain management regimens of the unit usually lead to a high degree of satisfactory pain control. In this context, it may have been difficult to improve upon an already established baseline of pain control and this limits generalisability of this trial to clinical practice in burn centres where opioid‐based pain management has not been optimised or is contraindicated in certain people.
While the included study did not show a reduction in opioid consumption or demand, or a difference in degree of participant satisfaction while the adjuvant use of intravenous lidocaine for pain relief during burn wound dressing changes was administered, the use of intravenous lidocaine as a form of pain relief at other stages during burn wound management remains unexplored in the form of RCTs. Given that several RCTs in a non‐burn context and case series involving people with burns highlight that intravenous lidocaine may lead to a reduction in participants' procedural and background pain levels, lead to less opiate requirements and consequently fewer associated complications, there is a need for larger RCTs to assess the utility of intravenous lidocaine in people with burns.
Authors' conclusions
Implications for practice.
The first update in 2012 identified one study for inclusion, and the conclusions from the original review were changed. This 2014 update did not identify any further eligible studies. The findings of this review update indicate that the use of intravenous lidocaine as an adjunctive analgesic agent in combination with morphine (patient‐controlled analgesia), although appearing to be well tolerated, confers little benefit on opioid consumption and demand, participant anxiety and satisfaction or time to rescue analgesia. However, these findings were based on one small single institution study where the intervention was implemented during procedural dressing changes. For this reason, it is impossible to draw any meaningful conclusions about the effect of intravenous lidocaine on burn‐associated pain at other stages in burn management, such as for pain control during the acute stages of presentation or in the management of chronic pain.
Implications for research.
In order to evaluate the effect of intravenous lidocaine as an analgesic agent in burn‐related injuries in a procedural context better, more research in the form of clinical randomised controlled trials (RCTs) should be undertaken with greater sample sizes and across multiple institutions. Future RCTs assessing the clinical effectiveness and safety should be undertaken at different stages of presentation in people with burns. Examples might include early in the person's admission to a burns centre and, where possible, before initiation of high‐dose opiate medications or for the treatment of chronic pain refractory to opiates or anti‐neuropathic medications.
What's new
| Date | Event | Description |
|---|---|---|
| 19 September 2019 | Amended | A new study identified (Abdelrahman 2019), although unlikely to change conclusions. See Published notes. |
| 30 April 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 17 October 2014 | Review declared as stable | This review will be assessed for updating in 2019, or sooner if new evidence becomes available. |
| 30 May 2014 | New search has been performed | New searches were run in December 2013, and Risk of bias tables were added. |
| 30 May 2014 | New citation required but conclusions have not changed | No new studies were found for inclusion in this review update. |
| 27 June 2012 | Amended | Contact details updated. |
| 29 May 2012 | Amended | The contact details of Patrick Mahar were amended for this update review conducted in April 2011. |
| 4 August 2011 | New citation required and conclusions have changed | This update includes one new study by Wasiak 2011 involving 45 participants. Previous readers of this review would benefit from re‐reading this review as there is now one small study included. |
| 4 August 2011 | New search has been performed | This review was brought up to date to April 2011; the original review was published in Issue 3, 2007. |
| 24 September 2010 | Amended | Contact details updated. |
| 30 October 2008 | Amended | Converted to new review format. |
Notes
March 2019
An updated search in March 2019 identified no potentially relevant studies likely to change the conclusions. We did identify one potentially relevant ongoing study (ClinicalTrials.gov Identifier: NCT02059902), although this study is unlikely to change the conclusions as it has a small number of participants (n = 28, at April 2019). Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
September 2019
In September 2019, the authors identified a potentially relevant study (Abdelrahman 2019), but this study was unlikely to change the conclusions. Therefore, the authors and editors judge that this review is still up to date, and we will assess the study for inclusion when we update the review.
Acknowledgements
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Burns] explode all trees
#2 burn*:ti,ab,kw (Word variations have been searched)
#3 thermal injur*:ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Lidocaine] this term only
#6 lidocaine or lignocaine:ti,ab,kw (Word variations have been searched)
#7 #5 or #6
#8 #4 and #7 from 2011 to 2013
Appendix 2. MEDLINE search strategy
Via Ovid for MEDLINE & Medline in Process April 2011 to 09/12/13
exp Burns/
burn*.mp.
thermal injur*.mp.
or/1‐3
Lidocaine/
(lidocaine or lignocaine).mp.
or/5‐64 and 7
(201104* or 201105* or 201106* or 201107* or 201108* or 201109* or 201110* or 201111* or 201112* or 2012* or2013*).ed.
8 and 9
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
or/11‐17
exp animals/
not humans.sh.
18 not 19
10 and 20
Key: mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier
via Ovid for the original review in 2007
exp BURNS/
(burn or burns or burned).mp
(burn$ or burns$ or burned$).au
burn.ti. or burn.ab. or burns.ti. or burns.ab. or burned.ti. or burned.ab.) and (burn$ or burns$ or burned$).au
2 not 3
4 or 5
burn.ti. or burn.ab. or burns.ti. or burns.ab. or burned.ti. or burned.ab.
2 not (1 or 3 or 7)
6 or 8
1 or 9
thermal injur$.mp
10 or 11
Lidocaine/
(lidocaine or lignocaine).mp
13 or 14
12 and 15
In addition, we checked the reference lists of the relevant trials and reviews. We did not contact current researchers in the field for unpublished data and ongoing trials.
Appendix 3. EMBASE search strategy
exp Burn/
burn*.mp.
thermal injur*.mp.4.
or/1‐3
Lidocaine
(lidocaine or lignocaine).mp
or/5‐6
4 and 7
(201104* or 201105* or 201106* or 201107* or 201108* or 201109* or 201110* or 201111* or 201112* or 2012* or2013*).dd.
8 and 9
random$.tw
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross‐over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
Crossover Procedure
double‐blind procedure.tw.
Randomized Controlled Trial/
Single Blind Procedure
11‐25
(animal/ or nonhuman/) not human/
26 not 27
10 and 28
Key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Wasiak 2011.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over study. Allocation concealment stated; method of randomisation stated; participants, staff and outcome assessors blind to treatment assignment and medication administration | |
| Participants | 45 participants with burns (mean % total body surface area 12.96%, range 3% to 55%) undergoing wound care procedures (i.e. dressing changes or debridement, or both) on 2 consecutive days following split skin‐graft surgery. Participants aged 16‐68 years | |
| Interventions | Intervention: lidocaine dose of 1.5 mg/kg/body weight followed by 2 boluses of 0.5 mg/kg at 5‐minute intervals followed by a continuous infusion Control: 0.9% sodium chloride administered at an equivalent volume, dose and rate to that of lidocaine |
|
| Outcomes | Verbal rating scale scores measured before, during and after the procedure; time to rescue analgesia; opioid requests and consumption using patient‐controlled analgesia; overall participant anxiety and level of satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized to one of two treatment sequence groups i.e. treatment A or treatment B, using block randomization" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of treatment regime was done using the opaque sealed envelope technique, in which envelopes with cards detailing which treatment regime were allocated to patients on the morning of each dressing" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects, staff and researchers were blinded to treatment assignments with all study medications identical in appearance. During the infusion, research staff administering the study medication was blind to the outcome assessments and the patient and research assistant were blinded to the study medication administration" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors did not report any loss to follow‐up participants for the primary outcome measures |
| Selective reporting (reporting bias) | Unclear risk | Authors did not provide overall participant satisfaction and anxiety scores |
| Size | High risk | < 50 participants per treatment arm |
| Other bias | Unclear risk | Not stated |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cassuto 2003 | Case report |
| Holthusen 2000 | Did not report on relevant primary outcome measures |
| Jönsson 1991 | Case series |
| Koppert 2004 | Did not report on relevant primary outcome measures |
| Mattsson 2000 | Did not report on relevant primary outcome measures |
Characteristics of studies awaiting assessment [ordered by study ID]
Abdelrahman 2019.
| Methods | Randomized controlled trial |
| Participants | 19 adult patients injured with burns of >10 total body surface area burned (TBSA%) |
| Interventions | Lidocaine infusion starting with a bolus dose (1 mg lidocaine/kg) followed by continuous infusion (180 mg lidocaine/hour) or a placebo infusion |
| Outcomes | VAS, TBSA%, time of enrolment to the study since the initial burn, duration of hospital stay, opioid consumption |
| Notes | This work was supported by, and done, at the Department of Hand Surgery, Plastic Surgery, and Burns, and the Linköping University, Linköping, Sweden. No specific grants from funding agencies in the public, commercial, or not‐for‐profit sectors were received. Conflicts of interest: none. |
Differences between protocol and review
For this update, we based 'Risk of bias' assessment on the methods outlined in The Cochrane Collaboration's 'Risk of bias' tool.
Contributions of authors
Jason Wasiak: designed and co‐ordinated the review. Extracted data and checked quality of data extraction. Undertook and checked quality assessment. Performed statistical analysis, interpreted data and checked the analysis. Completed first draft of the review update and advised on subsequent drafts. Made an intellectual contribution to the review and approved the final review prior to submission. Will be responsible for future updates of this review.
Hannah Tan: provided burns expertise and content to the review. Checked quality of data extraction and approved the final review prior to submission.
Heather Cleland: designed the review. Completed the first draft of the review and advised on subsequent drafts. Made an intellectual contribution to the review and approved the final review prior to submission. Performed previous work that was the foundation of the current review.
Patrick Mahar: extracted data and checked quality of data extraction. Made an intellectual contribution to the review and approved the final review prior to submission.
Siobhan McGuiness: extracted data and checked quality of data extraction. Made an intellectual contribution to the review and approved the final review prior to submission.
Stefan Danilla: provided burns expertise and content to the review. Checked quality of data extraction and approved the final review prior to submission.
For the first version of this review in 2007
Jason Wasiak: principal author, conception, guarantor of the review, responsible for updating future version of this review. Heather Cleland: co‐author, burn surgeon, content expert.
Sources of support
Internal sources
-
Victorian Adult Burns Service, Australia.
In‐kind support
External sources
No sources of support supplied
Declarations of interest
Mr Jason Wasiak and Miss Heather Cleland were co‐authors of the included study evaluated in this review article (Wasiak 2011).
The remaining authors have no known conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Wasiak 2011 {published data only}
- Wasiak J, Spinks A, Costello V, Ferraro F, Paul E, Konstantatos A, et al. Adjuvant use of intravenous lidocaine for procedural burn pain relief: a randomized double‐blind, placebo‐controlled, cross‐over trial. Burns 2011;37(6):951‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cassuto 2003 {published data only}
- Cassuto J, Tarnow P. Potent inhibition of burn pain without use of opiates. Burns 2003;29:163‐6. [DOI] [PubMed] [Google Scholar]
Holthusen 2000 {published data only}
- Holthusen H, Irsfeld S, Lipfert P. Effect of pre‐ or post‐traumatically applied i.v. lidocaine on primary and secondary hyperalgesia after experimental heat trauma in humans. Pain 2000;88(3):295‐302. [DOI] [PubMed] [Google Scholar]
Jönsson 1991 {published data only}
- Jönsson A, Cassuto J, Hanson B. Inhibition of burn pain by intravenous lignocaine infusion. Lancet 1991;338(8760):151‐2. [DOI] [PubMed] [Google Scholar]
Koppert 2004 {published data only}
- Koppert W, Brueckl V, Weidner C, Schmelz M. Mechanically induced axon reflex and hyperalgesia in human UV‐B burn are reduced by systemic lidocaine. European Journal of Pain 2004;8(3):237‐44. [DOI] [PubMed] [Google Scholar]
Mattsson 2000 {published data only}
- Mattsson U, Cassuto J, Tarnow P, Jonsson A, Jontell M. Intravenous lidocaine infusion in the treatment of experimental human skin burns ‐ digital colour image analysis of erythema development. Burns 2000;26(8):710‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdelrahman 2019 {published data only}
- Abdelrahman I, Steinvall I, Elmasry M, Sjoberg F. Lidocaine infusion has a 25% opioid‐sparing effect on background pain after burns: A prospective,randomised, double‐blind, controlled trial. Burns 2019;08:online. [DOI] [PubMed] [Google Scholar]
Additional references
Abelson 2002
- Abelson KS, Hoglund AU. Intravenous administered lidocaine in therapeutic doses increases the intraspinal release of acetylcholine in rats. Neuroscience Letter 2002;317(2):93‐6. [DOI] [PubMed] [Google Scholar]
Attal 2000
- Attal N, Gaude V, Brasseur L. Intravenous lidocaine in central pain: a double blind, placebo‐controlled, psychophysical study. Neurology 2000;54(3):564‐74. [DOI] [PubMed] [Google Scholar]
Dauber 2002
- Dauber A, Osgood PF, Breslau AJ. Chronic persistent pain after severe burns: a survey of 358 burns survivors. Pain Medicine 2002;3:6‐17. [DOI] [PubMed] [Google Scholar]
Edwards 1999
- Edwards AD. The role of systemic lidocaine in neuropathic pain management. Journal of Intravenous Nursing 1999;22(5):273. [PubMed] [Google Scholar]
Edwards 2007
- Edwards RR, Smith MT, Klick B, Magyar‐Russell G, Smit M, Weichman P. Symptoms of depression and anxiety as unique predictors of pain‐related outcomes following burn injury. Annals of Behavioural Medicine 2007;34(3):313‐22. [DOI] [PubMed] [Google Scholar]
Ferrante 1996
- Ferrante F, Michael MD, Paggioli J, Cherukuri S, Richard AG. The analgesic response to intravenous lidocaine in the treatment of neuropathic pain. Anesthesia and Analgesia 1996;82(1):91‐7. [DOI] [PubMed] [Google Scholar]
Finn 2004
- Finn J, Wright J, Fong J, Mackenzie E, Wood F, Leslie G, et al. A randomised crossover trial of patient controlled intranasal fentanyl and oral morphine for procedural wound care in adult patients with burns. Burns 2004;30(3):262‐8. [DOI] [PubMed] [Google Scholar]
Hand 2000
- Hand PJ, Stark RJ. Intravenous lignocaine infusions for severe chronic daily headache. Medical Journal of Australia 2000;172(4):157‐9. [DOI] [PubMed] [Google Scholar]
Harandi 2004
- Harandi AA, Esfandani A, Shakibaei F. The effect of hypnotherapy on procedural pain and state anxiety related to physiotherapy in women hospitalized in a burn unit. Contemporary Hypnosis 2004;21(1):28‐34. [Google Scholar]
Higgins 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Konstantatos 2009
- Konstantatos A, Angliss M, Costello V, Cleland H, Stafrace S. Predicting the effectiveness of virtual reality relaxation on pain and anxiety when added to PCA morphine in patients having burns dressing changes. Burns 2009;35(4):491‐9. [DOI] [PubMed] [Google Scholar]
McLeane 2001
- McLeane G. Intravenous infusion of lidocaine is not associated with changes in cardiovascular parameters: a study of 15 patients. The Pain Clinic 2001;13(1):83‐6. [Google Scholar]
Meyer 1981
- Meyer RP, Campbell JN. Myelinated nociceptive afferent account for hyperalgesia that follows a burn to the hand. Science 1981;213:1527‐9. [DOI] [PubMed] [Google Scholar]
Richardson 2009
- Richardson P, Mustard L. The management of pain in the burns unit. Burns 2009;35:921‐36. [DOI] [PubMed] [Google Scholar]
Summer 2007
- Summer GJ, Puntillo KA, Miakowski C, Green PG, Levine JD. Burn injury pain: the continuing challenge. Journal of Pain 2007;8(7):533‐48. [DOI] [PubMed] [Google Scholar]
Zor 2010
- Zor F, Ozturk S, Bilgin F, Isik S, Cosar A. Pain relief during dressing changes of major adult burns: ideal analgesic combination with ketamine. Burns 2010;36(4):501‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wasiak 2007
- Wasiak J, Cleland H. Lidocaine for pain relief in burn injured patients. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005622.pub2] [DOI] [PubMed] [Google Scholar]


