Figure 1.
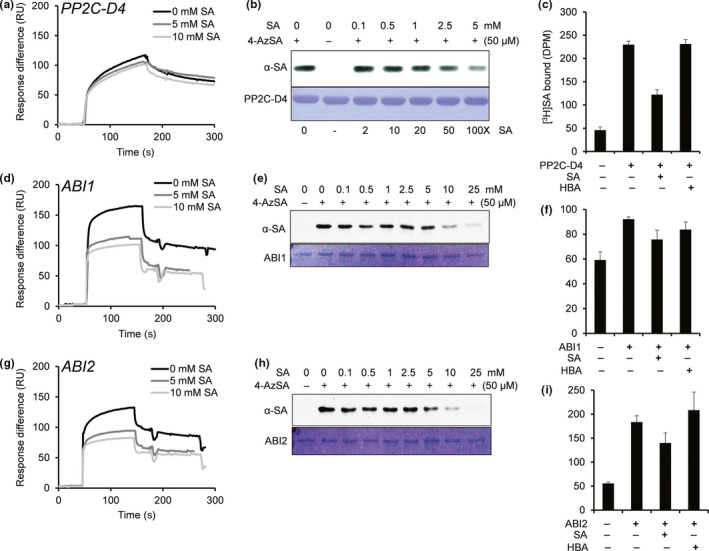
Several PP2Cs bind SA. (a, d, g) Sensorgrams for 1 μM of His6‐tagged PP2C‐D4 (At3g55050) (a), ABI1 (At4g26080) (d), and ABI2 (At5g57050) (g) in the absence (0 mM) or presence of two concentrations of SA (5 or 10 mM) using a 3AESA‐immobilized SPR sensor chip. Signals detected from a mock‐coupled control chip were subtracted. (b, e, h) Photo‐activated crosslinking of 50 ng of PP2C‐D4 (b), ABI1 (e), and ABI2 (h) to 4AzSA (50 μM) in the absence or presence of increasing amounts of SA was detected by immunoblotting using an α‐SA antibody. Reactions without 4AzSA served as negative controls. Proteins stained with Coomassie Brilliant Blue (CBB) served as the loading controls. (c, f, i) Binding of [3H]SA (200 nM) by 200 ng/μl PP2C‐D4 (c), ABI1 (f), and ABI2 (i) in the absence or presence of a 10,000‐fold excess of unlabeled SA was determined by size‐exclusion chromatography. Chromatography with [3H]SA in the absence of protein served as negative controls. Reactions with [3H]SA with excess of 4‐amino benzoic acid (4‐HBA), an inactive SA analog, served as negative controls for SA‐specific competitive inhibition. The experiments were independently repeated at least twice
