Abstract
GABAergic interneurons are emerging as prominent substrates in the pathophysiology of multiple neurodevelopmental disorders, including autism spectrum disorders, schizophrenia, intellectual disability, and epilepsy. Interneuron excitatory activity is influenced by 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptors (AMPARs), which in turn affects excitatory transmission in the central nervous system. Yet how dysregulation of interneuronal AMPARs distinctly contributes to the molecular underpinning of neurobiological disease is drastically underexplored. Contactin-associated protein-like 2 (CNTNAP2) is a neurexin-related adhesion molecule shown to mediate AMPAR subcellular distribution while calcium/calmodulin-dependent serine protein kinase (CASK) is a multi-functional scaffold involved with glutamate receptor trafficking. Mutations in both genes have overlapping disease associations, including autism spectrum disorders, intellectual disability, and epilepsy, thus suggesting converging perturbations of excitatory/inhibitory balance. Our lab has previously shown that CNTNAP2 stabilizes interneuron dendritic arbors through CASK and that CNTNAP2 regulates AMPAR subunit GluA1 trafficking in excitatory neurons. The interaction between these three proteins, however, has not been studied in interneurons. Using biochemical techniques, structured illumination microscopy (SIM) and shRNA technology, we first confirm that these three proteins interact in mouse brain, and then examined relationship between CNTNAP2, CASK and GluA1 in mature interneurons. Using SIM, we ascertain that a large fraction of endogenous CNTNAP2, CASK, and GluA1 molecules collectively colocalize together in a tripartite manner. Finally, individual knockdown of either CNTNAP2 or CASK similarly alter GluA1 levels and localization. These findings offer insight to molecular mechanisms underlying GluA1 regulation in interneurons.
Keywords: Autism spectrum disorders, Schizophrenia, Language disorders, AMPA receptors, Neuropsychiatric disorders, Cntnap2, Excitatory-inhibitory balance, Structured illumination microscopy, Dendrites, Mechanism
1. Introduction
AMPARs mediate excitatory transmission in glutamatergic and inhibitory neurons, making their function critical for proper brain activity. Structurally, AMPARs are tetramers consisting of different GluA1-GluA4 subunit combinations, with each subunit possessing unique biological, structural, and physiological properties [3]. Equally as intricate, there exists a growing list of AMPAR-associated proteins that dynamically regulate the receptor’s trafficking, composition, stability, and mobility in response to activity to ensure proper functional connectivity in synaptic circuits [13]. These regulatory mechanisms have been studied extensively in pyramidal cells, but are surprisingly understudied in inhibitory neurons, despite interneurons also being critical to proper circuit function [9].
Several recent studies have demonstrated that CNTNAP2 is involved in AMPAR-specific trafficking in pyramidal cells, as elimination of CNTNAP2 decreases synaptic GluA1, increases cytoplasmic GluA1 aggregates, and induces morphological changes in spiny synapses [2,42]. However, CNTNAP2′s role in AMPAR function within interneurons has not been explored despite the fact that Cntnap2 knockout mice have spontaneous seizures, decreased GABAergic interneuron density, and inhibitory-specific electrophysiological defects [19,31,44]. Moreover, CNTNAP2 has been shown to be highly abundant in the embryonic ganglionic eminences [31], consistent with a role in interneuron development. Similarly, the literature demonstrates that CASK interacts with CNTNAP2 [9,38], maintains pyramidal dendritic spines [6], and traffics N-methyl-d-aspartate receptors (NMDARs) [18,22] and AMPAR subunits [14]. While CASK has not been exclusively linked to interneuron function and is itself widely distributed in the brain [16], it is a multi-domain, multi-functional adaptor that can become specialized through interactions with specific molecules [4,15,29]. Hence, the role of CNTNAP2 on CASK function within interneurons is a topic that should be investigated.
Genetic variation in the CNTNAP2 gene, including copy number variations, exon deletions, truncations, single nucleotide variants, and polymorphisms have been associated with autism spectrum disorder, schizophrenia, intellectual disability, epilepsy, and language disorders[1,8,11,32,40,43]. Likewise, penetrant mutations within the CASK gene are causative for X-linked intellectual disability and have been associated with several cases of autism spectrum disorder [12,[25], [26], [27],35]. While both patient populations exhibit complex phenotypes, they share a common seizure comorbidity, which implicates a pathway convergence onto inhibitory circuits. Taken altogether, these observations suggest that complex CNTNAP2/CASKmutations may disrupt interneuron function through AMPAR dysregulation, but this theory has not yet been explored.
Here, we first uncover the physiological existence of CNTNAP2-CASK, CNTNAP2-GluA, and GluA1-CASK protein complexes in vivo. We then use SIM to show that CNTNAP2, CASK, and GluA1 most frequently exist in a tripartite complex in interneurons. Finally, transiently decreasing CNTNAP2 via shRNA alters GluA1 levels and localization in interneuron dendrites and somas – effects that are generally phenocopied by CASK knockdown. Our findings reveal clues to a possible postsynaptic mechanismthrough which CNTNAP2-CASK regulates GluA1 trafficking in interneurons.
2. Materials and methods
2.1. Antibodies and plasmids
Antibodies and plasmids used for the experiments are listed in Supplementary tables 1 and 2 respectively.
The pEGFP-N2 plasmid was purchased from Clontech (Mountain View, CA, USA). shRNA plasmids for CNTNAP2 (TG510300), CASK (TG516864), and scrambled controls (TR30015) were purchased from Origene (Rockwall, MD, USA), and were modified by replacing turboGFP with eGFP (restriction sites BglII and NotI). All interneurons were identified with either GABA staining [10] or morphological characteristics (i.e. multipolar, aspiny) when GABA staining was not permitted (see SIM Imaging and Analysis and Confocal Microscopy Imaging sections for more specifics).
2.2. Immunoprecipitation
Mouse cortices (3–5 months of age) were homogenized in immunoprecipitation buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.5% Triton X-100, with protease inhibitory cocktail from Roche, Basel, Switzerland) and solubilized for 1 h at 4 °C. Solubilized material was centrifuged at 20 000 g for 10 min at 4 °C and the supernatant was precleared with protein A/G (Thermo Fisher Scientific) for 30 min. Proteins in the precleared supernatant were then immunoprecipitated with 3 μg of antibody overnight at 4 °C, followed by a 1 h incubation with protein A/G the following day. Beads were then washed 3 times with IP buffer before adding 2x Laemelli buffer (Biorad, Hercules, CA, USA). Samples were analyzed by SDS-PAGE and western blotting using standard methods.
2.3. Neuronal culture and transfections
High density (300 000 cells/cm2) cortical neuron cultures were prepared from Sprague-Dawley rat E18 embryos as described previously [39]. Cortical neurons were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s recommendations (between 3–5 μg of DNA per plasmid, 4 μL of Lipofectamine 2000 per reaction) and the neurons were maintained in the feeding media [39] for either 3 (GFP only, endogenous staining experiments; 24–27 DIV), or 5 days (knockdown experiments; 21–26 DIV).
2.4. Immunocytochemistry
For total protein staining, neurons were first washed in phosphate buffered saline (1 × PBS) and fixed in 4% formaldehyde-sucrose-PBS for 15 min. Fixed neurons were permeabilized and blocked with 5% normal goat serum (NGS) and 0.3% Triton X-100 in 1 × PBS (30 min at 4 °C), followed by incubation of primary antibodies with 5% NGS in 1 × PBS (O/N, 4 °C). Coverslips were washed three times with PBS and incubated with the corresponding fluorophore-secondary antibodies with 5% NGS in 1 × PBS (Alexa Fluro 405, 488, 568, and/or 647; Thermo Fisher Scientific) for 1 h at room temperature (RT). The coverslips were then washed (3×) and mounted onto slides using ProLong Antifade reagent (Invitrogen, Carlsbad, CA, USA).
2.5. SIM imaging and analysis
Neurons were fixed, and immunocytochemistry was performed as described above. All analyzed interneurons were identified by morphology (i.e. multi-polar configuration and largely aspiny processes) here due to antibody constraints. Four-channel images were taken using a Nikon SIM microscope with a 100 × 1.49 NA objective (Cell Imaging Facility & Nikon imaging center, Northwestern University, Chicago, IL, USA) that allows a resolution range of 110–130 nm. Images were reconstructed (reconstruction parameters of 0.96, 1.19, and 0.17 for Illumination Modulation Contrast, High Resolution Noise Suppression, and Out of Focus Blur Suppression respectively were kept constant for all reconstructions), background-subtracted, and analyzed with FIJI as described previously [36]. Analysis of CNTNAP2-CASK-GluA1 patterns was accomplished using FIJI on single plane SIM images, by first selecting a 5 μm section of the dendrite with little contaminating fluorescence from adjacent cells. Next, the background was subtracted using rolling ball radius (50 pixels). Each channel was thresholded to create ROIs for 488, 568, and 647 within the selected segment of dendrite. ROIs of puncta that overlapped by ≥1 pixel were considered colocalized. We assessed CNTNAP2, CASK, and GluA1 puncta colocalization alone or within a two-or three-protein complex.
2.6. Confocal microscopy imaging
Confocal images of neurons were obtained as described previously [42]. Briefly, images were acquired by a Nikon (Amsterdam, Netherlands) C2 confocal microscope using a 63X oil immersion objective with numerical aperture (NA) = 1.4 with 0.4 μm z-stacks. All cells analyzed were interneurons, as determined by GABA antibody staining; images were acquired in the linear range of fluorescence intensity within either primary or secondary interneuron dendrites. The acquisition parameters were kept the same for all conditions and were analyzed using FIJI software. For puncta analysis, the “Analyze Particle” function was employed on a background-subtracted GFP-defined region-of-interest (ROI); puncta with areas less than 0.025 μm2 were excluded from the analysis. Puncta density was measured by number of puncta within a designated ROI divided by the ROI’s area. For immunofluorescence intensity, the mean pixel intensity of an ROI was measured; images were not background subtracted in this case.
2.7. Statistical analysis
All statistical tests were performed with GraphPad Prism (Version 7). Before analysis, data were first tested using D’Agostino’s Omnibus Normality Test and Pearson correlation in order to determine whether parametric or nonparametric tests were to be used. P values < 0.05 were considered significant.
3. Results
3.1. CASK, CNTNAP2, and GluA1 complex in vivo
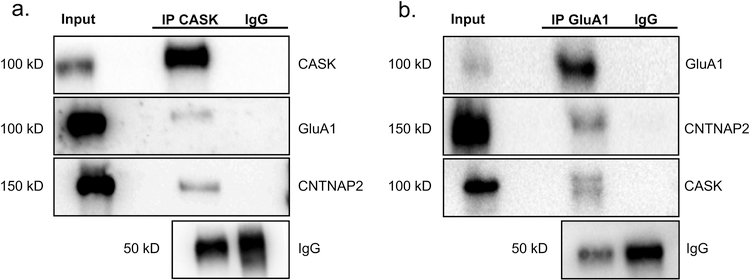
As in vitro studies show that CNTNAP2 and CASK interact in interneurons [10] and CNTNAP2 regulates GluA1 trafficking in excitatory neurons [42], we wanted to ascertain whether CNTNAP2 could physiologically interact with GluA1 and CASK in vivo. We first demonstrated that CASK could successfully pull down GluA1 and CNTNAP2 in adult mouse cortical homogenates (Fig. 1a). As supplementation, we also co-immunoprecipitated CNTNAP2 and CASK with GluA1 (Fig. 1b). These biochemical results support the literature and confirm that a fraction of CNTNAP2, CASK, and GluA1 participate in a biophysical multiprotein complex in vivo.
Fig. 1.
CNTNAP2 complexes with CASK and GluA1 in mouse cortical tissue. (a) Western blots of co-immunoprecipitation from mouse cortex using a CASK antibody. The CASK antibody successfully co-immunoprecipitated CNTNAP2 and GluA1. (b) Western blots of co-immunoprecipitation from mouse cortex using a GluA1 antibody. The GluA1 antibody successfully co-immunoprecipitated CNTNAP2 and CASK.
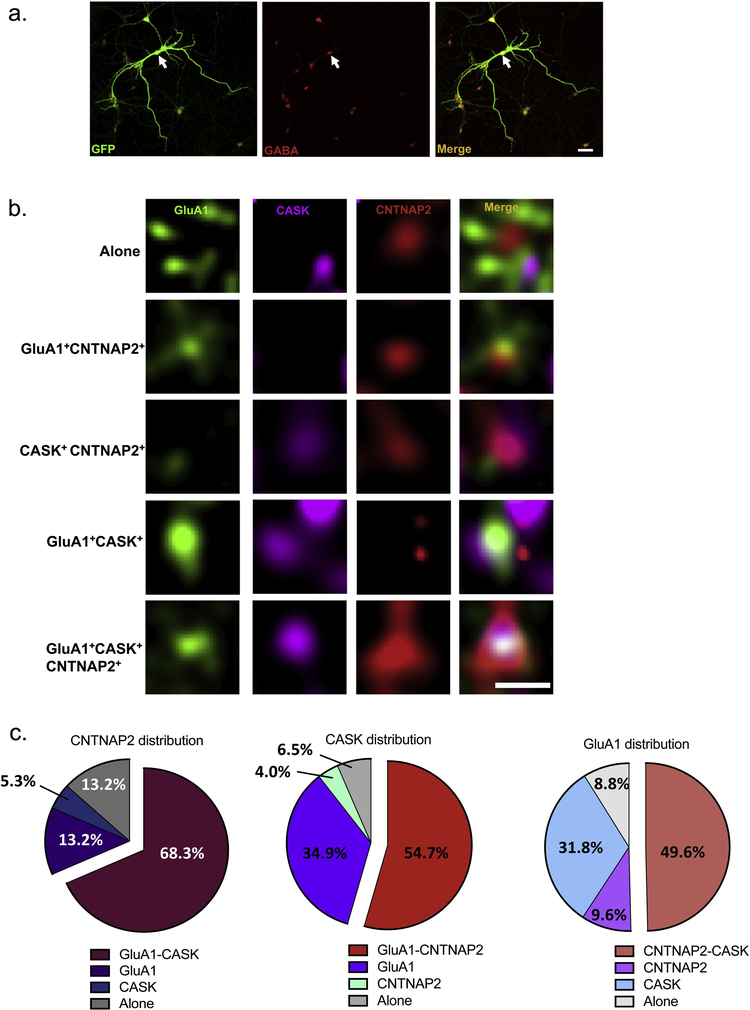
3.2. High resolution imaging reveals GluA1 in a complex with CNTNAP2 and CASK
Although immunoprecipitation studies indicated that CNTNAP2, CASK, and GluA1 interface in mouse cortical tissue, it is unclear whether this is through a single tripartite or multiple bipartite configurations. We therefore used SIM to examine the interaction patterns between the molecules in mature interneuronal dendrites (Fig. 2a). Endogenous CNTNAP2, CASK, and GluA1 were found either individually or complexed with one another in various combinations (Fig. 2b). Analytic studies of such compositions showed that the most common distribution was the tripartite configuration: 68.3% of CNTNAP2 puncta colocalized with GluA1-CASK molecules, 54.7% of CASK puncta colocalized with GluA1-CNTNAP2 molecules, and 49.6% of GluA1 puncta colocalized with CNTNAP2-CASK molecules (Fig. 2c). Interestingly, individual CNTNAP2 (13.2%), CASK (6.5%), and GluA1 (8.8%) molecules were rarely observed (Fig. 2c).
Fig. 2.
SIM reveals CNTNAP2, CASK, GluA1 most frequently form a tripartite complex. (a) Representative low-magnification confocal image of an interneuron, confirmed by co-staining with a GABA antibody (scale bar = 50 μ m). (b) Representative high-resolution SIM images displaying various distributions of endogenous CNTNAP2, CASK, and GluA in the dendrites of 27 DIV interneurons (scale bar = 0.5 μ m). (c) Categorization of the patterns seen in (b) (n = 98–135 total puncta from 12 branches and 3 independent experiments).
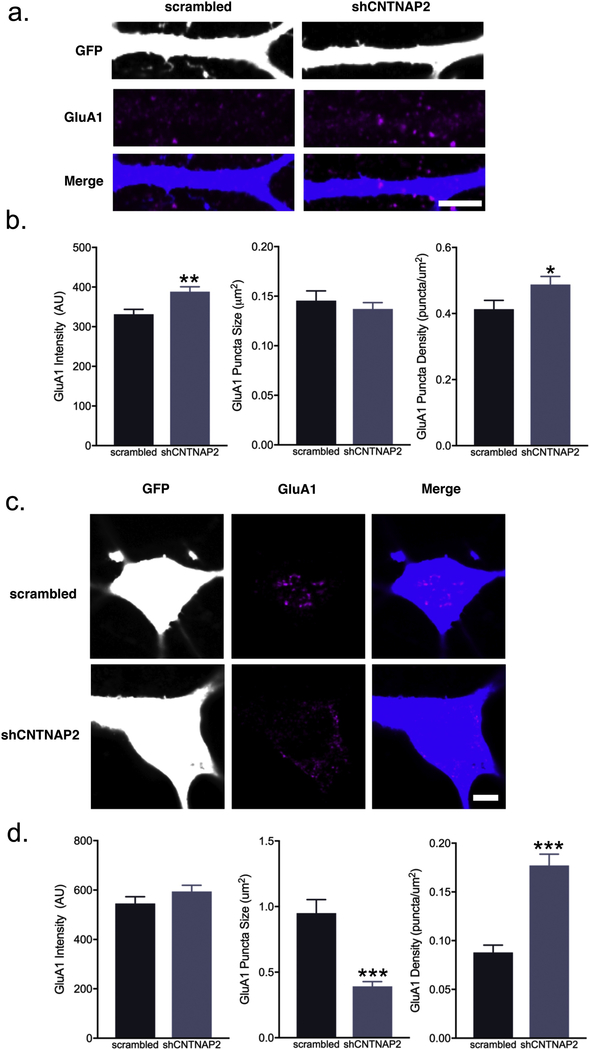
3.3. CNTNAP2 knockdown alters dendrite/soma total GluA1 patterns
Given that our data suggests CNTNAP2, CASK, and GluA1 colocalize, we sought to identify functional consequences of this interaction. We next assessed whether manipulation of CNTNAP2 would affect GluA1 expression patterns in cultured interneurons. We found that CNTNAP2 knockdown (21–26 DIV) in rat mature interneurons (Supplemental Fig. 1a) led to a surprising increase in average total GluA1 dendrite intensity (scrambled: 331.4 ± 12.2 AU vs. shCNTNAP2: 388.5 ± 12.1 AU; Fig. 3a–b) and puncta density (scrambled: 0.41 ± 0.02 puncta/μm2 vs. shCNTNAP2: 0.48 ± 0.02 puncta/μm2; Fig. 3a–b). Puncta size, however, was unaltered in either condition (scrambled: 0.15 ± 0.01 μm2 vs. shCNTNAP2: 0.14 ± 0.006 μm2; Fig. 3a–b).
Fig. 3.
CNTNAP2 knockdown in interneurons leads to alterations of GluA1 content and clustering. (a) Representative confocal images and (b) quantification of total GluA1 average intensity, puncta size, and puncta area in scrambled or shCNTNAP2-treated (21–26 DIV) rat interneuron dendrites at 26 DIV (scale bar = 5 μ m; intensity: n = 72 branches; puncta size and density: n = 68–71 branches from 5 independent experiments). (c) Representative confocal images and (d) quantification of GluA1 average intensity, puncta size, and puncta area in scrambled or shCNTNAP2-treated (21–26 DIV) rat interneuron somas at 26 DIV (scale bar = 5 μ m; intensity: n = 40 cells; puncta size and density: n = 39–40 cells from 5 independent experiments). Values are means ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001; Student’s t-test (d, intensity), Mann-Whitney test (b; d, puncta size and density).
Soma GluA1 patterns had distinct patterns compared to dendrites: soma total GluA1 intensity was unchanged between scrambled and shCNTNAP2 interneurons (scrambled: 545.7 ± 27.28 AU vs. shCNTNAP2: 594 ± 25.0 AU; Fig. 3c–d), while GluA1 puncta density (scrambled: 0.08 ± 0.008 puncta/μm2 vs. shCNTNAP2: 0.18 ± 0.01 puncta/μm2; Fig. 3c–d) was increased and puncta size (scrambled: 0.95 ± 0.10 μm2 vs. shCNTNAP2: 0.39 ± 0.04 μm2; Fig. 3c–d) decreased in the knockdown.
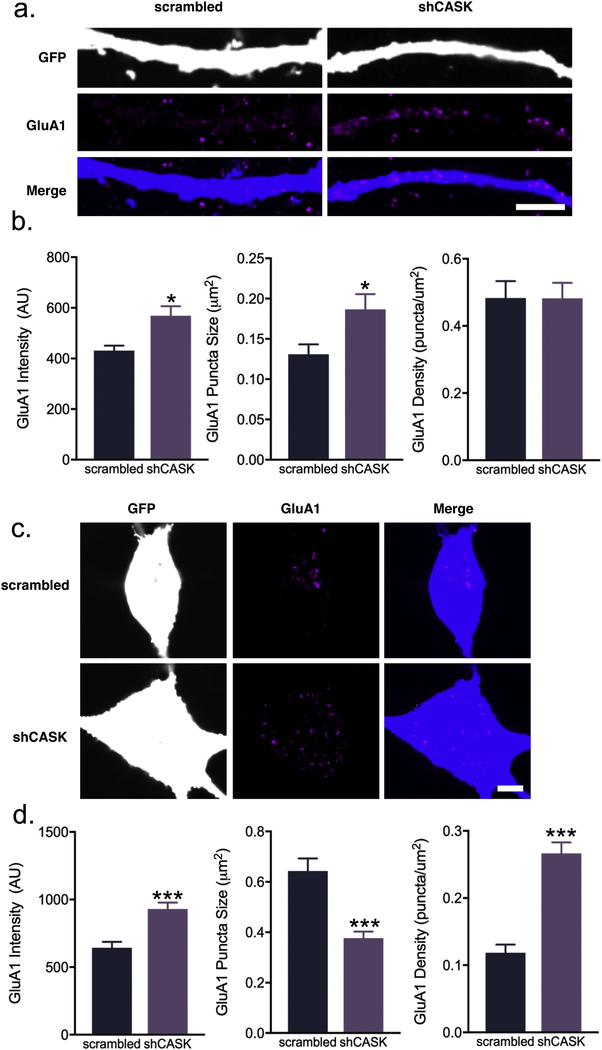
3.4. CASK knockdown results in altered total GluA1 patterns in interneuron soma/dendrites
Next, we evaluated the effects of CASK knockdown (Supplemental Fig. 1b) on GluA1 levels and localization. We report that shCASK dendrites had increased total GluA1 intensity (scrambled: 430.9 ± 20.29 AU vs. shCASK 568.9 ± 37.57 AU; Fig. 4a–b) and puncta size (scrambled: 0.131 ± 0.01 μm2 vs. shCASK: 0.186 ± 0.01 μm2; Fig. 4a–b), but not puncta density (scrambled: 0.483 ± 0.05 puncta/μm2 vs. shCASK: 0.482 ± 0.04 puncta/μm2; Fig. 4a–b).
Fig. 4.
CASK knockdown in interneurons leads to alterations of GluA1 content and clustering. (a) Representative confocal images and (b) quantification of GluA1 average intensity, puncta size, and puncta area in scrambled or shCASK-treated (21–26 DIV) rat interneuron dendrites at 26 DIV (scale bar = 5 μ m; intensity: n = 35–36 branches; puncta size and density: n = 33 branches from 3 independent experiments). (c) Representative confocal images and (d) quantification of GluA1 average intensity, puncta size, and puncta area in scrambled or shCASK-treated (21–26 DIV) rat interneuron somas at 26 DIV (scale bar = 5 μ m; n = 20–21 cells from 3 independent experiments). Values are means ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001; Student’s t-test (b, intensity; d), Mann-Whitney test (b, puncta size and density).
On the other hand, GluA1 clusters within the soma of shCASK interneurons appear much more widely dispersed but smaller compared to scrambled cells, as reflected in the quantifications: higher average total GluA1 intensity (scrambled: 643.5 ± 43.48 AU vs. shCASK: 930.3 ± 47.23 AU; Fig. 4c–d), smaller puncta size (scrambled: 0.643 ± 0.04 μm2vs. shCASK: 0.376 ± 0.026 μm2; Fig. 4c–d), and greater puncta density (scrambled: 0.118 ± 0.012 puncta/μm2 vs. shCASK: 0.266 ± 0.016 puncta/μm2; Fig. 4c–d). Thus, shCASK interneurons recapitulates some aspects of shCNTNAP2 interneurons, providing further evidence GluA1 is regulated by the CNTNAP2-CASK complex.
4. Discussion
Interneurons must possess a perfect balance of distinctive arborization morphology, unique electrophysiological properties, and precise positioning to successfully entrain pyramidal counterparts to fire at particular synchronous rhythms required for proper excitatory transmission [9,17]. Failure of such procedures can lead to devastating consequences for proper information transfer and ultimately cause behavior abnormalities. Consequently, inhibitory neurons have been implicated as one of the major substrates in the pathophysiology of complex psychiatric diseases such as schizophrenia, autism, and intellectual disability [23]. Indeed, multiple studies have uncovered molecules involved in the control of interneuronal glutamatergic synapses [5,30,41], suggesting the presence of cell-specific mechanisms of synapse plasticity, although the literature on this is relatively sparse.
Here we investigate how CNTNAP2, a molecule highly expressed in interneurons [24,44], may regulate GluA1 in inhibitory cell types. Utilizing multiple convergent methods – namely SIM, co-immunoprecipitation, and shRNA – we found that CNTNAP2 forms a complex with CASK and GluA1 in vivo and the three proteins colocalize frequently in tripartite patterns within interneuron dendrites. Transient knockdown of either CNTNAP2 or CASK leads to overall elevated GluA1, suggesting CNTNAP2 and CASK may synergistically regulate GluA1 abundance in interneurons. Since CNTNAP2 interacts directly with CASK at the membrane and CASK has been shown to traffic NMDARs/AMPARs, we speculate that CNTNAP2 binds GluA1 through CASK. Function-wise, both CASK or CNTNAP2 knockdown cause GluA1 to lose focalized distribution. This observation, along with the finding that genetic ablation of Cntnap2 causes GluA1 aggregates to form [42], provides strong evidence CASK and CNTNAP2 work in conjunction to traffic GluA1.
On the other hand, GluA1 dendrite patterns in CASK or CNTNAP2 knockdown interneurons are not entirely similar, suggesting different mechanisms in either scenario. For instance, CASK can interact with AMPAR subunits through other mediators like GRIP1 [14], while CNTNAP2 associates with protein 4.1, another CASK-interacting scaffold molecule involved with AMPAR trafficking [4,7,21]. Thus sudden loss of CNTNAP2 or CASK in interneuronal dendrites, while both affecting GluA1, may not have the same exact effects. Furthermore, we have previously shown that CASK and CNTNAP2 alter dendritic arborization morphology in inhibitory neurons [10]. Thus, it is also possible that changes in the CNTNAP2-CASK interactions destabilize neuronal structure which in turn alters synaptic GluA1 localization [20,28,45].
Patients with highly penetrant CNTNAP2 or CASK mutations have overlapping symptomology, particularly in regards to seizures [12,33,37,40], thereby suggesting common pathways involving excitatory/inhibitory balance. From previous data, we speculate that CNTNAP2-CASK may serve as part of a cellular pathway that converges upon interneuron regulation. Here, we show initial evidence that GluA1 may be one of the downstream targets of CNTNAP2-CASK and that disruption of CNTNAP2 or CASK may contribute to neurodevelopmental diseases by interfering with GluA1 quantity and localization. Interestingly, we previously found reduced levels of GluA1 in the spines of Cntnap2 knockout pyramidal neurons, which directly conflicts with our current findings. This discrepancy may be due to differences in molecular abundance between different cell types, as CNTNAP2 is more highly expressed in interneurons [24]. Another explanation could be the lack of compensation in shRNA knockdown compared to knockout models [34]; for example, knockdown, but not knockout, of CNTNAP2 causes decreased pyramidal cell arborization [2,10]. While follow-up studies are critical to tease apart these subtle differences, our findings provide the first definite molecular link between CNTNAP2, CASK, and GluA1.
Supplementary Material
Acknowledgements
This work was supported by grants NS100785 and MH097216 from the NIH-NIMH to P.P and F30MH096457to R.G. SIM imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Structured illumination microscopy was performed on a Nikon N-SIM system, purchased through the support of NIH 1S10OD016342-01. We thank Xi Chao for help with figure illustrations.
Footnotes
Conflict of interest
The authors declare no competing financial conflict of interests.
Appendix A. Supplementary data
Four supplementary files are available for this article
References
- [1].Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH, Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene, Am. J. Hum. Genet 82 (2008) 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC, Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 18120–18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anggono V, Huganir RL, Regulation of AMPA receptor trafficking and synaptic plasticity, Curr. Opin. Neurobiol 22 (2012) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Biederer T, Sudhof TC, CASK and protein 4.1 support F-actin nucleation on neurexins, J. Biol. Chem 276 (2001) 47869–47876. [DOI] [PubMed] [Google Scholar]
- [5].Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF, Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons, Nat. Neurosci 13 (2010) 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chao HW, Hong CJ, Huang TN, Lin YL, Hsueh YP, SUMOylation of the MAGUK protein CASK regulates dendritic spinogenesis, J. Cell Biol. 182 (2008) 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA, Protein 4.1B associates with both Caspr/ paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres, Eur. J. Neurosci 17 (2003) 411–416. [DOI] [PubMed] [Google Scholar]
- [8].Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van Kessel AG, Wijmenga C, Ophoff RA, Veltman JA, CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy, Mol. Psychiatry 13 (2008) 261–266. [DOI] [PubMed] [Google Scholar]
- [9].Gao R, Penzes P, Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders, Curr. Mol. Med 15 (2015) 146–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gao R, Piguel NH, Melendez-Zaidi AE, Martin-de-Saavedra MD, Yoon S, Forrest MP, Myczek K, Zhang G, Russell TA, Csernansky JG, Surmeier DJ, Penzes, CNTNAP2 stabilizes interneuron dendritic arbors through CASK, Mol. Psychiatry (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gregor A, Albrecht B, Bader I, Bijlsma EK, Ekici AB, Engels H, Hackmann K, Horn D, Hoyer J, Klapecki J, Kohlhase J, Maystadt I, Nagl S, Prott E, Tinschert S, Ullmann R, Wohlleber E, Woods G, Reis A, Rauch A, Zweier C, Expanding the clinical spectrum associated with defects in CNTNAP2 and NRXN1, BMC Med. Genet 12 (2011) 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hackett A, Tarpey PS, Licata A, Cox J, Whibley A, Boyle J, Rogers C, Grigg J, Partington M, Stevenson RE, Tolmie J, Yates JR, Turner G, Wilson M, Futreal AP, Corbett M, Shaw M, Gecz J, Raymond FL, Stratton MR, Schwartz CE, Abidi FE, CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes, Eur. J. Hum. Genet 18 (2010) 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Henley JM, Wilkinson KA, Synaptic AMPA receptor composition in development, plasticity and disease, Nat. Rev. Neurosci 17 (2016) 337–350. [DOI] [PubMed] [Google Scholar]
- [14].Hong CJ, Hsueh YP, CASK associates with glutamate receptor interacting protein and signaling molecules, Biochem. Biophys. Res. Commun 351 (2006) 771–776. [DOI] [PubMed] [Google Scholar]
- [15].Hsueh YP, Wang TF, Yang FC, Sheng M, Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2, Nature 404 (2000) 298–302. [DOI] [PubMed] [Google Scholar]
- [16].Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M, Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses, J. Cell Biol 142 (1998) 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang ZJ, Di Cristo G, Ango F, Development of GABA innervation in the cerebral and cerebellar cortices, Nat. Rev. Neurosci 8 (2007) 673–686. [DOI] [PubMed] [Google Scholar]
- [18].Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de Silva T, Montgomery JM, Garner CC, Green WN, SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway, Nat. Neurosci 12 (2009) 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jurgensen S, Castillo PE, Selective dysregulation of hippocampal inhibition in the mouse lacking autism candidate gene CNTNAP2, J. Neurosci 35 (2015) 14681–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Koleske AJ, Molecular mechanisms of dendrite stability, Nat. Rev. Neurosci 14 (2013) 536–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL, Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation, Nat. Neurosci 12 (2009) 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin EI, Jeyifous O, Green WN, CASK regulates SAP97 conformation and its interactions with AMPA and NMDA receptors, J. Neurosci 33 (2013) 12067–12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marin O, Interneuron dysfunction in psychiatric disorders, Nat. Rev. Neurosci 13 (2012) 107–120. [DOI] [PubMed] [Google Scholar]
- [24].Mo A, Mukamel EA, Davis FP, Luo C, Henry GL, Picard S, Urich MA, Nery JR, Sejnowski TJ, Lister R, Eddy SR, Ecker JR, Nathans J, Epigenomic Signatures of Neuronal Diversity in the Mammalian Brain, Neuron 86 (2015) 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moog U, Bierhals T, Brand K, Bautsch J, Biskup S, Brune T, Denecke J, de Die-Smulders CE, Evers C, Hempel M, Henneke M, Yntema H, Menten B, Pietz J, Pfundt R, Schmidtke J, Steinemann D, Stumpel CT, Van Maldergem L, Kutsche K, Phenotypic and molecular insights into CASK-related disorders in males, Orphanet J. Rare Dis 10 (2015) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moog U, Kutsche K, Kortum F, Chilian B, Bierhals T, Apeshiotis N, Balg S, Chassaing N, Coubes C, Das S, Engels H, Van Esch H, Grasshoff U, Heise M, Isidor B, Jarvis J, Koehler U, Martin T, Oehl-Jaschkowitz B, Ortibus E, Pilz DT, Prabhakar P, Rappold G, Rau I, Rettenberger G, Schluter G, Scott RH, Shoukier M, Wohlleber E, Zirn B, Dobyns WB, Uyanik G, Phenotypic spectrum associated with CASK loss-of-function mutations, J. Med. Genet 48 (2011) 741–751. [DOI] [PubMed] [Google Scholar]
- [27].Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, Christian SL, Ullmann R, Kuechler A, Haas CA, Flubacher A, Charnas LR, Uyanik G, Frank U, Klopocki E, Dobyns WB, Kutsche K, Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum, Nat. Genet 40 (2008) 1065–1067. [DOI] [PubMed] [Google Scholar]
- [28].Niell CM, Meyer MP, Smith SJ, In vivo imaging of synapse formation on a growing dendritic arbor, Nat. Neurosci 7 (2004) 254–260. [DOI] [PubMed] [Google Scholar]
- [29].Olsen O, Moore KA, Fukata M, Kazuta T, Trinidad JC, Kauer FW, Streuli M, Misawa H, Burlingame AL, Nicoll RA, Bredt DS, Neurotransmitter release regulated by a MALS-liprin-alpha presynaptic complex, J. Cell Biol 170 (2005) 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, Cameron HA, Murata Y, Colonnese MT, Worley PF, McBain CJ, Pentraxins Coordinate Excitatory Synapse Maturation and Circuit Integration of Parvalbumin Interneurons, Neuron 90 (2016) 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH, Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits, Cell 147 (2011) 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rodenas-Cuadrado P, Ho J, Vernes SC, Shining a light on CNTNAP2: complex functions to complex disorders, Eur. J. Hum. Genet 22 (2014) 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodenas-Cuadrado P, Pietrafusa N, Francavilla T, La Neve A, Striano P, Vernes SC, Characterisation of CASPR2 deficiency disorder–a syndrome involving autism, epilepsy and language impairment, BMC Med. Genet 17 (2016) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY, Genetic compensation induced by deleterious mutations but not gene knockdowns, Nature 524 (2015) 230–233. [DOI] [PubMed] [Google Scholar]
- [35].Seto T, Hamazaki T, Nishigaki S, Kudo S, Shintaku H, Ondo Y, Shimojima K, Yamamoto T, A novel CASK mutation identified in siblings exhibiting developmental disorders with/without microcephaly, Intractable Rare Dis. Res 6 (2017) 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, Myczek K, Radulovic J, Swanson GT, Penzes P, Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses, Neuron 84 (2014) 399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smogavec M, Cleall A, Hoyer J, Lederer D, Nassogne MC, Palmer EE, Deprez M, Benoit V, Maystadt I, Noakes C, Leal A, Shaw M, Gecz J, Raymond L, Reis A, Shears D, Brockmann K, Zweier C, Eight further individuals with intellectual disability and epilepsy carrying bi-allelic CNTNAP2 aberrations allow delineation of the mutational and phenotypic spectrum, J. Med. Genet 53 (2016) 820–827. [DOI] [PubMed] [Google Scholar]
- [38].Spiegel I, Salomon D, Erne B, Schaeren-Wiemers N, Peles E, Caspr3 and caspr4, two novel members of the caspr family are expressed in the nervous system and interact with PDZ domains, Mol. Cell. Neurosci 20 (2002) 283–297. [DOI] [PubMed] [Google Scholar]
- [39].Srivastava DP, Woolfrey KM, Penzes P, Analysis of dendritic spine morphology in cultured CNS neurons, J. Vis. Exp (2011) e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH, Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2, N. Engl. J. Med 354 (2006) 1370–1377. [DOI] [PubMed] [Google Scholar]
- [41].Tao Y, Chen YJ, Shen C, Luo Z, Bates CR, Lee D, Marchetto S, Gao TM, Borg JP, Xiong WC, Mei L, Erbin interacts with TARP gamma-2 for surface expression of AMPA receptors in cortical interneurons, Nat. Neurosci 16 (2013) 290–299. [DOI] [PubMed] [Google Scholar]
- [42].Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schurmann B, Fleming HJ, Fawcett-Patel JM, Bach A, Jang S, Peles E, Kim E, Penzes P, Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 6176–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA, Tourette G, Syndrome Association International Consortium for, CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder, Genomics 82 (2003) 1–9. [DOI] [PubMed] [Google Scholar]
- [44].Vogt D, Cho KKA, Shelton SM, Paul A, Huang ZJ, Sohal VS, Rubenstein JLR, Mouse Cntnap2 and human CNTNAP2 ASD alleles cell autonomously regulate PV+ cortical interneurons, Cereb. Cortex (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu GY, Zou DJ, Rajan I, Cline H, Dendritic dynamics in vivo change during neuronal maturation, J. Neurosci 19 (1999) 4472–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.