Fig 1. Products of CYP6DJ1 assays with (+)-(4R)-limonene and products detected in female beetles after treatment with (+)-(4R)-limonene.
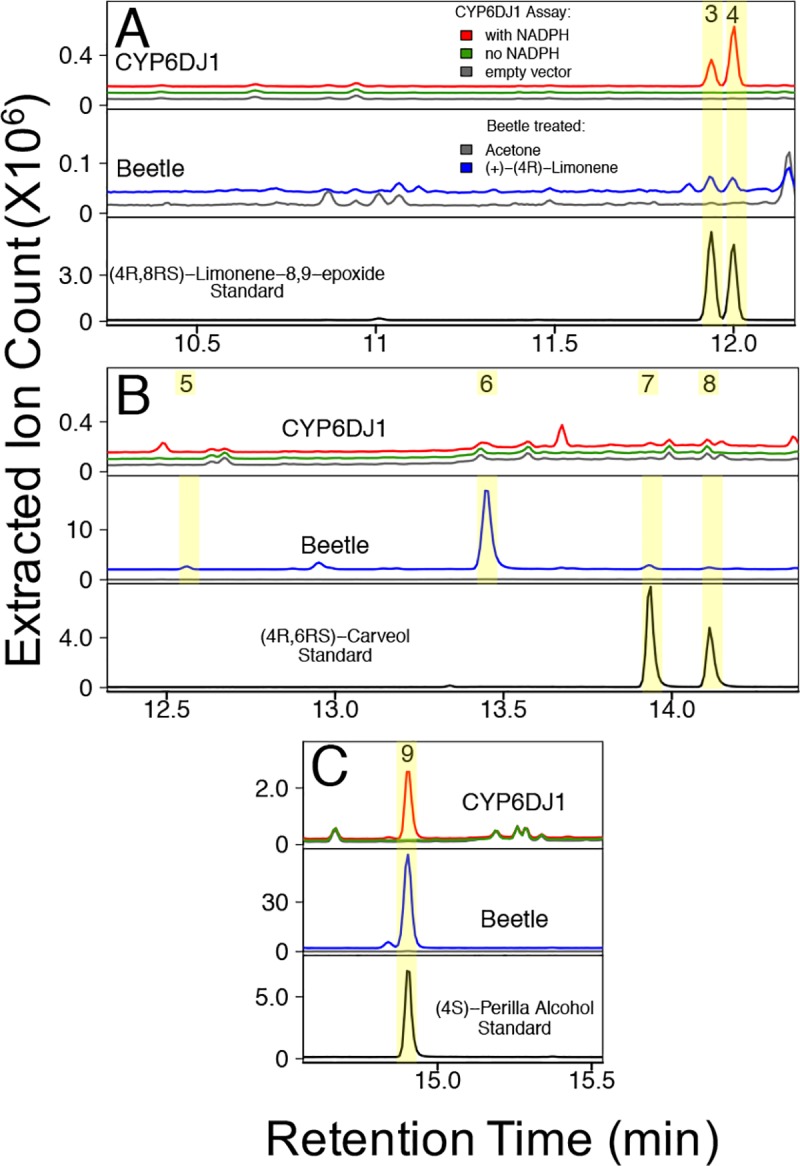
Panels A-C show different regions of the same chromatograms of CYP6DJ1 assays and extracts of beetles treated with (+)-(4R)-limonene. (A) (4R,8R)-Limonene-8,9-epoxide (peak 3) and (4R,8S)-limonene-8,9-epoxide (peak 4) were produced by CYP6DJ1 in in vitro assays with (+)-(4R)-limonene as substrate and were detected in beetles treated with (+)-(4R)-limonene. (B) Unidentified product (peak 5), (+)-trans-(3R,4S)-isopiperitenol (peak 6), (+)-cis-(4S,6S)-carveol (peak 7) and (+)-trans-(4S,6R)-carveol (peak 8) were present in beetles treated with (+)-(4R)-limonene. (C) (4R)-Perilla alcohol (peak 9) was produced in CYP6DJ1 assays with (+)-(4R)-limonene and was detected in beetles treated with (+)-(4R)-limonene. Chromatograms are shown with the total of the extracted ions 91, 94, 108, 109, 119, 121, 137, 152 m/z. Retention indices and mass spectra of peaks 1–10 are shown in S2 Table and S3 Fig.
