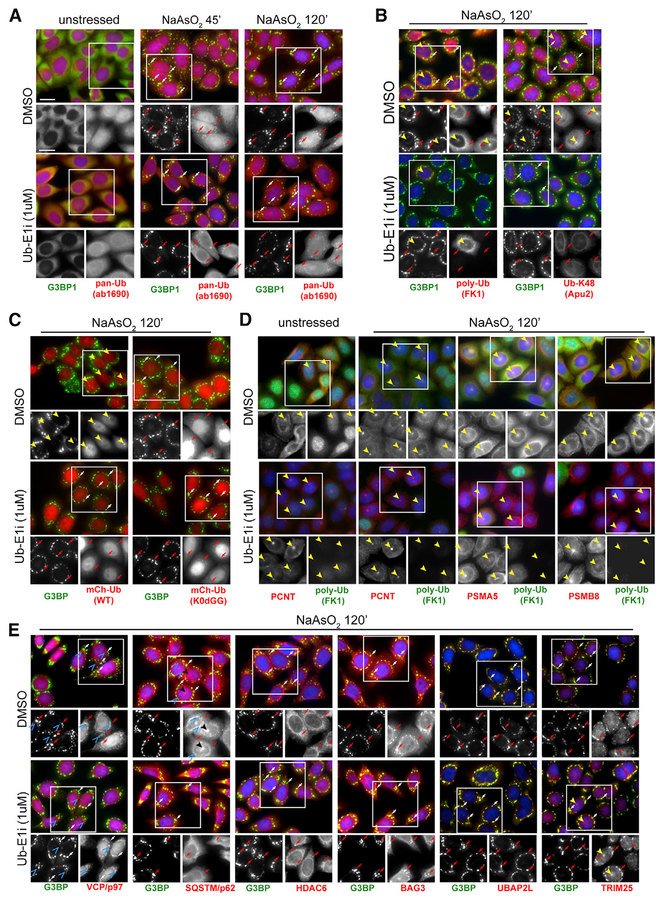
Figure 4. Unconjugated Ubiquitin Localizes to SGs in a UAE-Independent Manner.
(A–E) Immunofluorescence staining of HeLa cells treated with NaAsO2 (250 μM) for 0’, 45’, or 120’ prior to fixation (A) or pretreated with DMSO or UbE1i (1 μ M) for 90’, followed by treatment with NaAsO2 (250 mM) for 120’ prior to fixation (B–E). Cells were stained with antibodies against G3BP1 (A–E), pan-ubiquitin (A), polyubiquitin (B and D), centrosome marker pericentrin (D), proteasome subunits (D), VCP/p97 (E), SQSTM/p62 (E), HDAC6 (E), UBAP2L (E), or TRIM 25 (E). (C) Cells stably expressing either mCherry-tagged wild-type ubiquitin (mCh-Ub-WT) or mCherry-tagged ubiquitin, in which all internal lysine residues were mutated to arginine and the C-terminal diglycine residues were removed (mCh-Ub-K0DGG [K0dGG]) were stained with antibodies against G3BP1. G3BP1-positive SGs are indicated by arrows (both red and white). G3BP1-negative perinuclear foci are indicated by solid yellow arrowheads. G3BP1-negative punctate signal for VCP/p97 and SQSTM/p62 is indicated by blue open arrowheads. Images from Ub-E1i-treated cells in all panels were taken at the same exposure time and acquisition settings in the ubiquitin channel as those for images from DMSO-treated cells. Nuclei were stained using DAPI. Scale bars, 20 μ m in all panels.
See also Figure S4.