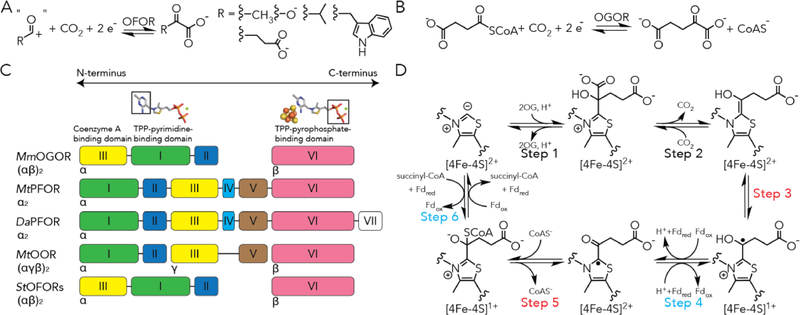
Figure 1. 2-oxoacid:ferredoxin oxidoreductases (OFORs) and the domain arrangements of structurally characterized OFORs.
(A) General scheme of OFOR reactions. (B) 2-oxoglutarate:ferredoxin oxidoreductase (OGOR) reversibly reduce carbon dioxide and succinyl-CoA into 2-oxoglutarate. (C) The domain arrangements of structurally characterized OFORs. Domain III is a coenzyme A binding domain. Domain I binds the pyrimidine moiety of thiamine pyrophosphate (TPP). Domain VI binds the pyrophosphate of TPP and a [4Fe-4S] cluster. Domain V, which is absent in MmOGOR, adapts a ferredoxin fold that binds two [4Fe-4S] clusters in PFOR and OOR. (D) The proposed reaction mechanism of carbon fixation and 2-oxoglutarate oxidation by OGOR based on studies from MtPFOR.14,19.20 Steps involving Fd-based electron transfer are labeled with teal, and those involving internal electron transfer are labeled with red.