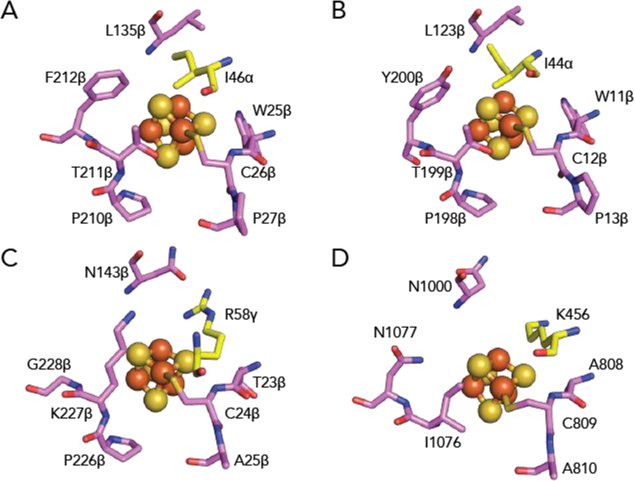
Figure 4. The environment of the proximal [4Fe-4S] cluster in structurally characterized OFORs.
(A) In MmOGOR, the proximal cluster is surrounded by hydrophobic residues. The hydrophobic interactions include Leu135β and two loops formed by cluster-ligating motifs, 25βWCP27β and 209βCPTF212β. Additionally, Ile46α, which is a positively charged residue in PFOR and OOR, is contributed by domain III. (B) In StOFOR1 (PDB ID: 5B4825), the proximal cluster is also surrounded by hydrophobic residues. The hydrophobic interactions include Leu123β, two neighboring loops that are part of cluster-ligating motifs, 11βWCP13β, and 197βCPTY200β, and Ile44α from domain III. (C) In MtOOR (PDB ID: 5C4I12), a conserved positively-charged arginine from domain III, Arg58γ, resides next to the proximal cluster. Other residues surrounding the proximal cluster are smaller in comparison to those of MmOGOR and StOFORs. (D) In MtPFOR (PDB ID: 6CIN19), a conserved positively-charged lysine from domain III, Lys456, is within hydrogen-bonding distance with the proximal cluster. Other residues surrounding the proximal cluster are smaller in comparison to those of MmOGOR and StOFORs. Domain coloring as in Figure 1A.