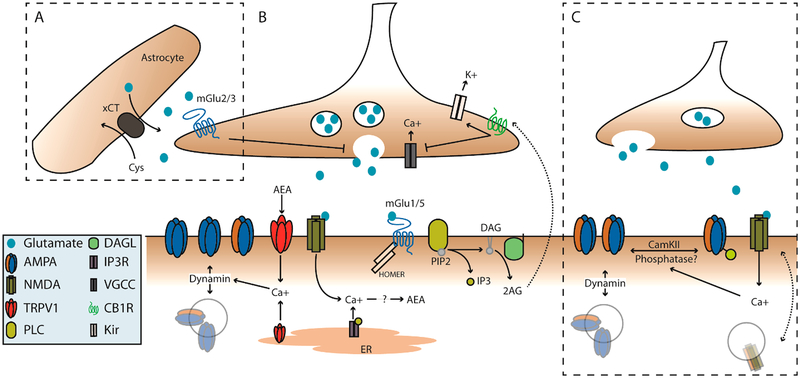
Figure 1.
mGlu and NMDA plasticity mechanisms coordinate pre- and postsynaptic function of NAc glutamatergic synapses. (A) Extrasynaptic glutamate homeostasis couples to group II mGlu activation. Extra-synaptic glutamate is tightly regulated by astrocytic cysteine-glutamate antiporter (xCT). High extracellular glutamate activates group II mGlu receptors to decrease presynaptic release. (B) Postsynaptic activation of group-1 mGlu receptors is known to recruit PLC to generate IP3 and DAG. DAG can be further cleaved by DAGL to a free fatty acid and 2-arachidonyl glycerol (2AG), which can signal to presynaptic CB1Rs. Activation of CB1Rs can act via inhibition of VGCCs and/or activation of presynaptic potassium channels to decrease vesicular release. Additionally, activation group-1 mGlu receptors can also induce a calcium dependent synthesis of anandamide (AEA), which likewise acts on CB1Rs but also activates TRPV1 channels. Activation of TRPV1 at the membrane or on the ER induces a dynamin-dependent internalization of AMPA receptors. (C) NMDA dependent LTD and LTP. Endogenous glutamate/glycine binding and concurrent depolarization activates NMDA receptors allowing an influx of calcium which can couple to downstream phosphatase/kinase cascades. These likely include CamKII and calcineurin, which can phosphorylate/dephosphorylate AMPA receptors, respectively. This contributes to their insertion or removal from the postsynaptic density. However, this mechanism is not well-defined in the NAc.