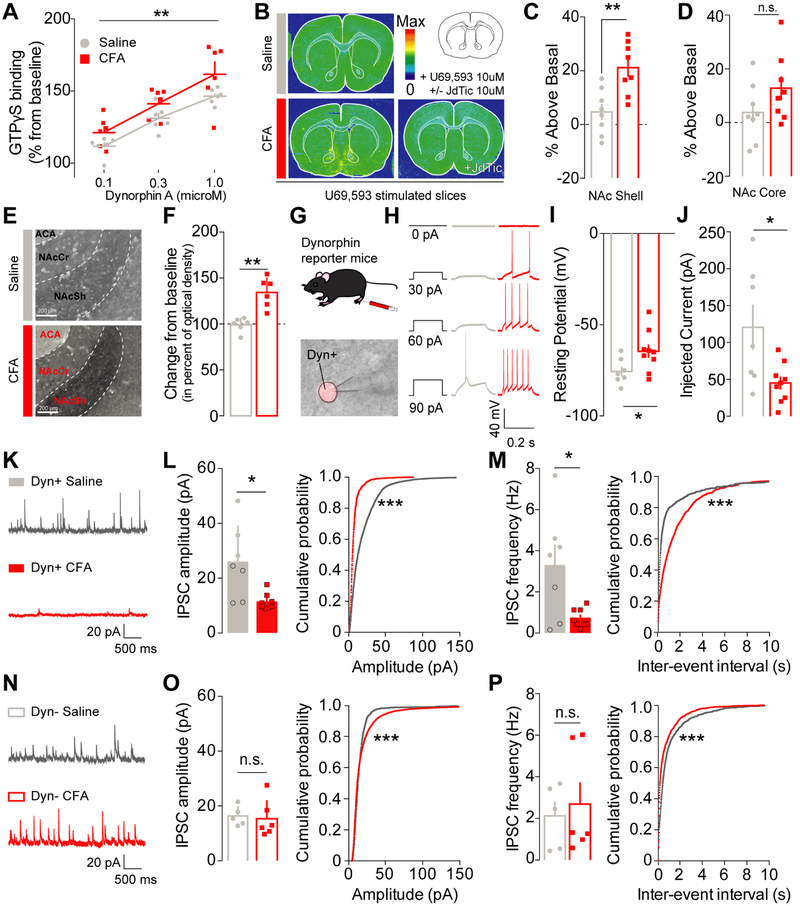
Figure 3: Inflammatory pain increases KOR functional activity and recruits dynorphin-containing neurons in the NAcShell through a disinhibition mechanism.
(A) Dynorphin A stimulation dose-dependently increases GTPγS incorporation in NAc tissue from CFA- (n=6) and saline-injected rats (n=6) (two-way mixed-model ANOVA: dose: F1, 30= 8.717, p=0.0066). GTPγS incorporation is significantly higher in CFA-treated animals suggesting an increase in KOR functional activity (two-way mixed-model ANOVA: pain effect: F2, 30 = 29.98, p<0.0001). (B) Representative GTPγS autoradiography of slices incubated with U69,593 in saline (top) and CFA +/− JdTic (bottom) injected animals. (C) In conditions of pain, KOR functional activity was increased in the NAc shell (unpaired two-way t-test: p=0.0027, n=8). (D) but not in the NAc core (unpaired two-way t-test: p=0.1450, n=8). (E) Representative pictures of dynorphin A expression in the NAcShCS in either saline (top) and CFA-injected animals (bottom). ACA: Anterior Commisure, NAcSh: Nucleus Accumbens Shell; NAcCr: Nucleus Accumbens Core. (F) Dynorphin A content in the NAcShCS is increased after CFA-induced inflammation (Mann-Whitney test for unpaired values; p=0.0022, n=6). (G) Schematic representation of electrophysiology methodology. Lower panel : representative picture a patch pipet onto the somatic area of a Ai14+ dynorphin neuron in the vNACsh. (H) Representative traces of current-response from vNacSh dynorphin neurons obtained from either saline or CFA mice. (I) CFA-injected animals display a higher resting membrane potential (unpaired two-way t-test: p=0.032, ncells/animals=7-9/4), and (J) a lower rheobase (unpaired two-way t-test: p=0.019, ncells/animals=7-9/4). (K) Representative traces of sIPSCs from dyn+ neurons. (L) Amplitude and (M) frequency of sIPSC onto dyn+ neurons are decreased in conditions of inflammatory pain as compared to saline control (frequency: two–tailed t-test for unpaired values p=0.0406; amplitude: two-tailed Mann-Whitney for unpaired values p=0.0140, ncells/animals=7-8/4). The cumulative probability plots demonstrate a significant shift towards smaller and less frequent events in dynorphin+ neurons after CFA (Kolmogorov-Smirnov test p<0.0001, ncells/animals=7-8/4). (N) Representative traces of sIPSCs from Dyn- neurons. (O) Neither the mean amplitude or (P) frequency of sIPSCs onto dyn- neurons are affected by inflammatory pain (frequency: two-tailed Mann-Whitney for unpaired values p=0.6277; amplitude: two–tailed Mann-Whitney for unpaired values p=0.4242, ncells/animals=5-6/4). A plot of the cumulative probability revealed a significant shift towards larger and more frequent events in dynorphin negative neurons after CFA (Kolmogorov-Smirnov test p<0.0001, ncells/animals=5-6/4).