Table 1.
Optimization of the reaction conditions
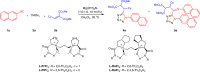
| |||||||
|---|---|---|---|---|---|---|---|
| 4a | 5a | ||||||
| entry | Ligand (L) | Mg(OTf)2: L | T (°C) | Yield (%)a | e.r.b | Yield (%)a | e.r.b |
| 1 | L-PrPr 2 | 1.0:1.0 | 35 | 29 | 82:18 | 53 | 92.5:7.5 |
| 2 | L-PiPr 2 | 1.0:1.0 | 35 | – | – | 97 | 82:18 |
| 3 | L-RaPr 2 | 1.0:1.0 | 35 | 38 | 83.5:16.5 | 46 | 96:4 |
| 4 | L-RaPr 3 | 1.0:1.0 | 35 | 36 | 72:28 | 43 | 86.5:13.5 |
| 5 | L-RaPr 2 | 1.5:1.0 | 35 | – | – | 88 | 94:6 |
| 6 | L-RaPr 2 | 1.0:1.5 | 35 | 53 | 88:12 | 25 | 95.5:4.5 |
| 7c | L-RaPr 2 | 1.4:1.0 | 30 | – | – | 91 | 94.5:5.5 |
| 8d | L-RaPr 2 | 1.0:1.5 | −40 | 57 | 94.5:5.5 | – | – |
| 9d,e | L-RaPr 2 | 1.0:1.5 | −40 | 73 | 95:5 | – | – |
| 10f | L-RaPr 2 | 1.0:1.5 | −40 then −20 | 91 | 95:5 | – | – |
Unless otherwise noted, all reactions were carried out with 1a (0.10 mmol), 2a (0.12 mmol), 3a (0.10 mmol), and Mg(OTf)2/L-RaPr2 (1.0:1.0, 10 mol%) in CH2Cl2 (1.0 mL) at 35 °C for 3 h
aIsolated yield
bDetermined by CSP-HPLC analysis
cCarried out with 1a (0.20 mmol), 2a (0.24 mmol), 3a (0.20 mmol), and Mg(OTf)2/L-RaPr2 (1.4:1.0, 10 mol%) in CH2ClCH2Cl (1.0 mL) at 30 °C for 3 h
d−40 °C for 7 days
eWith 5 Å MS (10 mg) as the additive
fCarried out with 1a (0.10 mmol), 2a (0.15 mmol), 3a (0.15 mmol), 5 Å MS (10 mg), and Mg(OTf)2/L-RaPr2 (1.0/1.5, 10 mol%) in CH2Cl2 (1.0 mL) at −40 °C for 2 days, then −20 °C for 3 days
