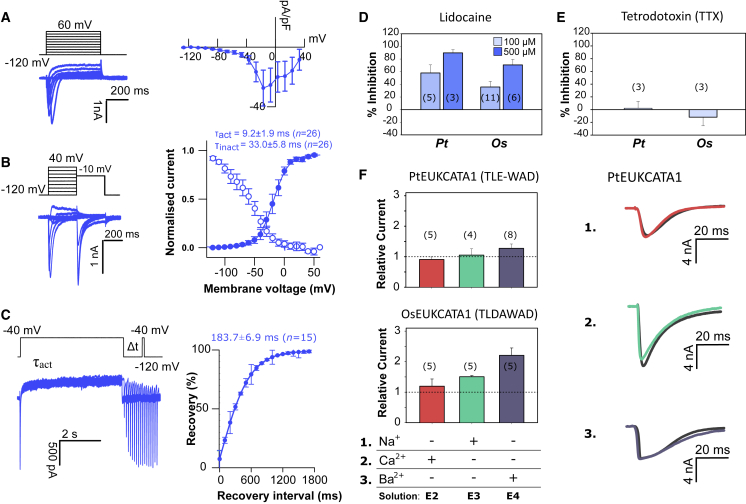
Figure 2.
Diatom EukCatAs Are Rapid-Depolarization-Activated Na+- and Ca2+-Permeable Channels
(A) Typical current traces for HEK293 cells expressing PtEUKCATA1 in response to membrane depolarization. Average peak current-voltage (I-V) curve (right), where current was normalized to cell capacitance. Peak current: −27.4 ± 12.0 pA/pF (error bars, SEM; n = 8).
(B) Steady-state inactivation: representative current traces (left) used to obtain steady-state inactivation curves (right) for PtEUKCATA1 (n = 17). Average normalized data were fitted using the Boltzmann equation (see STAR Methods) for both activation (solid circles) and steady-state inactivation (open circles) curves. Error bars: SEM (see Table S1 for time constants).
(C) Recovery from inactivation; superimposed currents obtained by a double-pulse protocol using a varying interval (Δt) between the two voltage pulses (left). Holding potential was −120 mV and the test pulse 40 mV for 100 ms, and the recovery pulse of −40 mV for 5 s was applied between 100 ms and 1,700 ms after the test pulse. The peak currents elicited by the recovery pulse were normalized in order to construct the recovery curve. A single exponential was fitted to the averaged normalized recovery curve yielding τ for recovery (first order exponential fit) of 650 ± 33.5 ms (error bars, SEM; n = 3).
(D and E) Impact of lidocaine (D) and tetrodotoxin TTX (10 μM; E) on diatom EukCatA channels: PtEUKCATA1 and OsEUKCATA1. Plots show mean % inhibition (relative to control current); error bars: SEM, n shown in parentheses. Inhibition of native HEK cell Na+ currents by TTX (10 μM) is also shown. TTX had no statistically significant impact on currents PtEUKCATA1 and OsEUKCATA1 (Student’s t test: p values: >0.5; n = 3).
(F) Mean peak currents generated following external cation substitution relative to the control external solution for PtEUKCATA1 (SF: TLE-WAD) and OsEUKCATA1 (SF: TLDAWAD; with representative traces for PtEUKCATA1, right; relative current was calculated by dividing the peak current in the test solution by the peak current in the control extracellular solution E1; Table S2). Replacement of extracellular Na+ with NMDG did not affect EukCatA currents. Similarly, removal of Ca2+ had no effect. Replacement of Ca2+ with Ba2+ (in the absence of Na+) slightly enhanced the EukCatA currents. Error bars: SEM; nl shown in parentheses. Extracellular and intracellular (pipette) solutions are given in Table S2.
See also Figure S1, Methods S1, and Tables S1 and S2.