Figure 6.
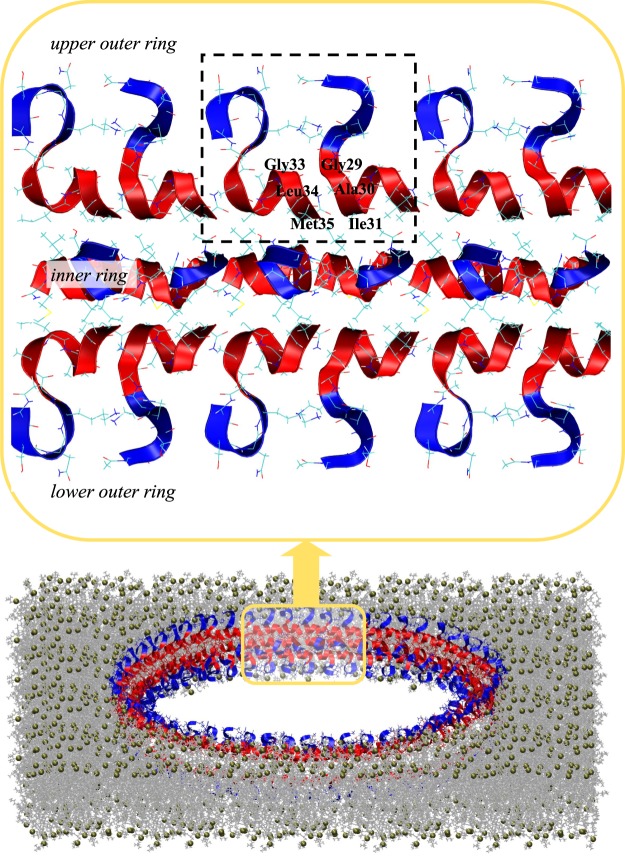
A hypothetical structure of Aβ25-35 annular oligomer or pore in the lipid bilayer formed by three concentric rings of peptides. There are two outer rings placed in the upper and lower bilayer leaflets interacting via their hydrophobic C-termini (in red) with the bilayer core. The peptides N-termini (in blue) occur next to the bilayer surface interacting with the lipid headgroups. The third inner ring is shifted closer to the pore center and its polar N-termini are directed toward the pore center creating its polar lining. The inset magnifies the six peptides from each of the three rings. As building blocks the rings utilize ID Aβ25-35 dimers enclosed by dashed lines. The amino acids participating in the dimer parallel aggregation interface are identified. The bilayer representation follows that used in Fig. 1c. The oligomer has a diameter of 20 nm as measured by AFM36.