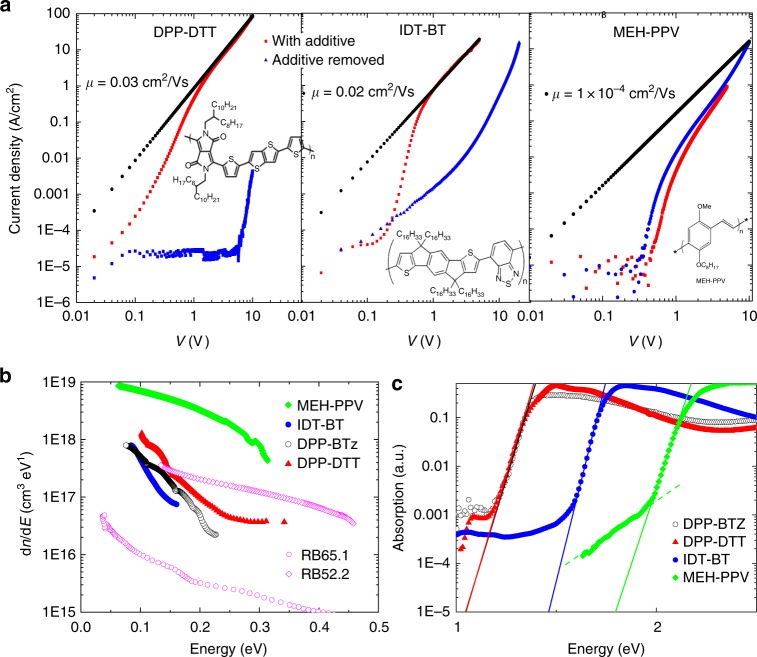
Fig. 3.
Trap removal through solvent additives in a range of polymers. a Log–Log J–V characteristics for the polymers diketopyrrolo-pyrrole-dithiophene-thienothiophene (DPP-DTT) (d = 130 nm, left panel), indacenodithiophen-co-benzothiadiazole (IDT-BT) (d=220 nm, central panel), and poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV) (d = 60 nm, right panel). Red squares correspond to devices with a solvent additive (residual 1,2-dichlorobenzene (DCB) solvent), blue triangles correspond to the same device after removal of the solvent additive, and black circles are fits to the space charge-limited conduction (SCLC) region with corresponding mobilities shown. The polymer’s molecular structures are shown as an inset. b Density of trap states extracted from SCLC and optical spectroscopy: dn/dE values extracted using temperature-dependent SCLC spectroscopy for the polymers MEH-PPV, IDT-BT, poly[[2,5-bis(2-octadecyl)-2,3,5,6-tetrahydro-−3,6-diketopyrrolo[3,4-c]pyrrole-1,4-diyl]-alt-(2-octylnonyl)-2,1,3-benzotriazole] (DPP-BTz) and DPP-DTT, as well as for single crystals of rubrene (data from ref. 23); all polymer data was obtained for devices with a solvent additive in place. c Absorption coefficient of IDT-BT, DPP-DTT, DPP-BTz, and MEH-PPV films, measured by photothermal deflection spectroscopy (PDS). Solid lines represent exponential tail fits for extraction of the Urbach energies