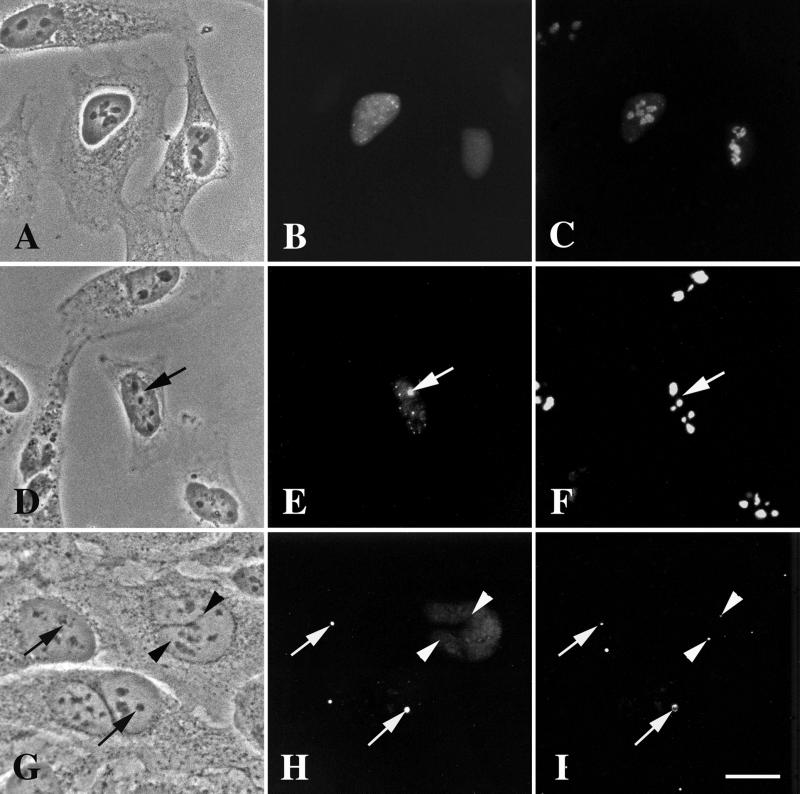
Figure 8.
Expression of the carboxyl-terminal truncation, DmNopp140ΔRGG, in transfected HeLa cells. (A–C) GFP-DmNopp140ΔRGG failed to localize to intact phase-dark nucleoli. The cells were counterstained with the S4 anti-human fibrillarin antibody and a rhodamine-conjugated secondary antibody (C). Most of the endogenous fibrillarin remained associated with the nucleoli. (D–F) In approximately half the transfected cells GFP-DmNopp140ΔRGG caused nucleoli to partition into phase-light (precisely matched arrows D–F) and phase-dark regions, reminiscent of the effects caused by GFP-DmNopp140. The cells were counterstained with anti-human nucleolin and a rhodamine-conjugated secondary antibody (F). Nucleolin localized only within the phase-dark regions that were completely devoid of GFP-DmNopp140ΔRGG. (G–I) Transfected HeLa cells again expressed GFP-DmNopp140ΔRGG (H). Some cells displayed a diffuse distribution of GFP-DmNopp140ΔRGG, whereas other cells displayed partitioned nucleoli with DmNopp140ΔRGG localizing to the phase-light regions (precisely aligned arrows in G and H). The cells were counterstained with the R288 antiserum directed against human coilin (I). Arrowheads show endogenous CBs that did not contain GFP-DmNopp140ΔRGG. Conversely, arrows show phase-light regions that appear to contain endogenous coilin. Bar (A–I), 20 μm.