Table 1.
Exploration of pyrrolidin-2-one ring substitutions in the 1 scaffold.
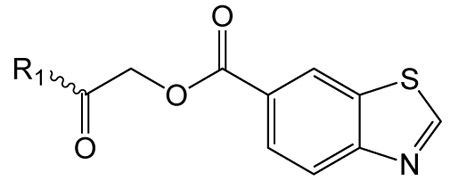 |
||||
|---|---|---|---|---|
| compd | R1 | IC50 ± SD [μM]a |
Hill coeff b | % Activity ESIc |
| 1 |  |
4.0 ± 0.2 | 2.34 ± 0.19 | 1.40 ± 0.1 |
| 1a | 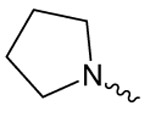 |
17.1 ± 1.2 | 2.57 ± 0.40 | 6.04 ± 0.46 |
| 1b | 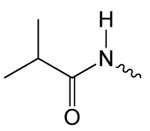 |
3.6 ± 0.6 | 1.65 ± 0.38 | 61.74 ± 4.31 |
| 1c |  |
47.2 ± 8.4 | 1.46 ± 0.26 | 9.01 ± 0.78 |
| 1d | 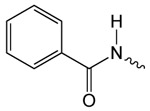 |
48.5 ± 3.8 | 2.89 ± 0.32 | 14.64 ± 0.74 |
| 1e | 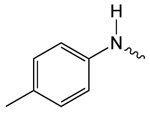 |
8.5 ± 0.7 | 2.93 ± 0.48 | 3.09 ± 0.25 |
| 1f | 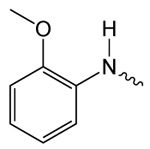 |
10.7 ± 2.5 | 1.48 ± 0.13 | 59.42 ± 1.61 |
All experiments to determine IC50 values were run with at least duplicates at each compound dilution, IC50 values were averaged when determined in two or more independent experiments.
Hill coefficient obtained upon fitting the concentration-response curves.
DGC activity of the enzyme-substrate-inhibitor complex.
