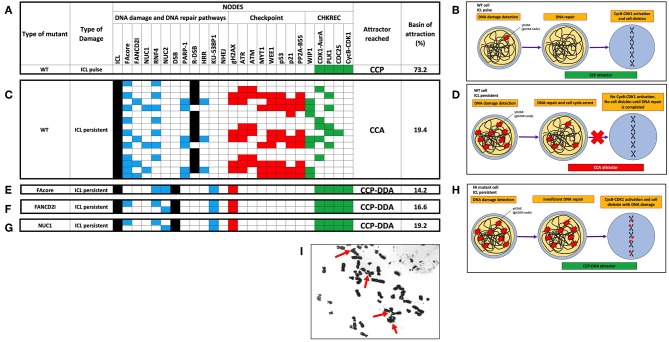
Figure 2.
Selected attractors showing that the FA-CHKREC BNM recapitulates the current knowledge on the FA/BRCA pathway and the response of cell cycle checkpoints to DNA damage. (A) In the WT network detection and repair of an ICL pulse by the FA/BRCA pathway allows activation of the CHKREC nodes, reaching a fixed-point CCP attractor. Cell division strictly requires CycB-CDK1 node activation. (B) Schematics of the DNA damage detection and repair cellular processes activated upon DNA damage induction. WT cells efficiently detect and repair small pulses of DNA damage and reach the CCP attractor, which implies cell division. (C) In presence of a persistent ICL the WT network remains in a cyclic CCA attractor, is not able to activate the CycB-CDK1 node, until the source of damage is removed. (D) Schematics showing that WT cells will remain arrested in a CCA attractor, implying a blocking in cell division, until the DNA damage source is removed and DNA damage is fully repaired. (E-G) Representative FA mutants activate the CHKREC full process despite persistent DNA damage (ICLs and DSBs), arriving to a fixed-point attractor, that we denominate the CCP-DDA (Cell Cycle Progression with DNA Damage Adaptation) attractor. (E) FAcore mutant, (F) FANCD2I mutant, (G) NUC1 mutant. (H) Schematics showing that despite unrepaired DNA damage FA cells reach cell division (CCP-DDA attractor). (I) Representative metaphase from the FA cell line VU817 (FANCA mutant, FAcore mutant) showing unrepaired DNA damage in the form of chromosome breakage that reached cell division (red arrows). Only attractors are shown. Nodes in the simulations are grouped by color, according to functional categories: DNA damage in black, DNA repair pathways in blue, Checkpoint in red and CHKREC in green. Inactive nodes are colorless, whereas active nodes are colored according to their functional category. Refer to Supplementary Material S1 to see the whole trajectories to attractors of these and other mutants.