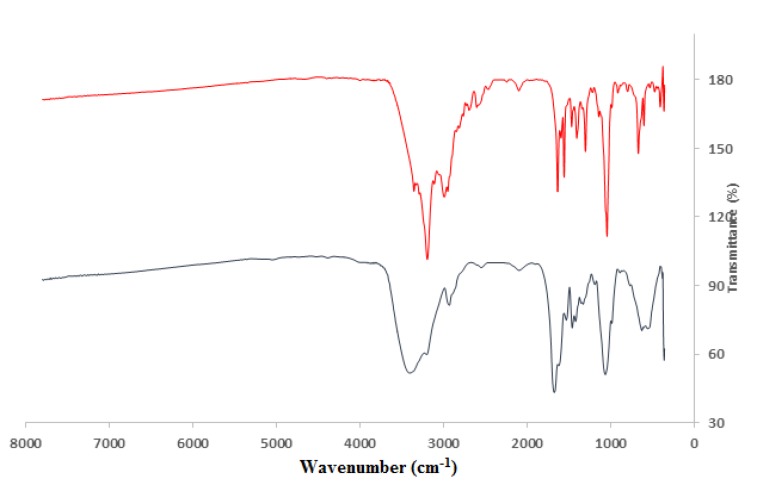
Figure 3.
Fourier transform infrared (FTIR) spectrum of antifungal peptides derived from (upper line) Bacillus pumilus DFAR8 and (lower line) Bacillus pumilus ZED17 strains. The infrared spectrum of antifungal compound were drawn with Excell 2012 software and showed characteristic absorption valleys at 1671,1616,1630, 1553, 1296 cm-1 indicating the secondary structure of peptides include α-helix, β-sheet and turn structures. Valleys that result from C-H stretching (2928 cm-1) and (3197 cm-1) indicate the presence of aliphatic and aromatic chain respectively. The valley absorbance at 3999 cm-1 represents strong H-bonding of acid, amine & amid groups, and the valley at 3350 cm-1 is related to NH stretching of terminal amine