FIGURE 8.
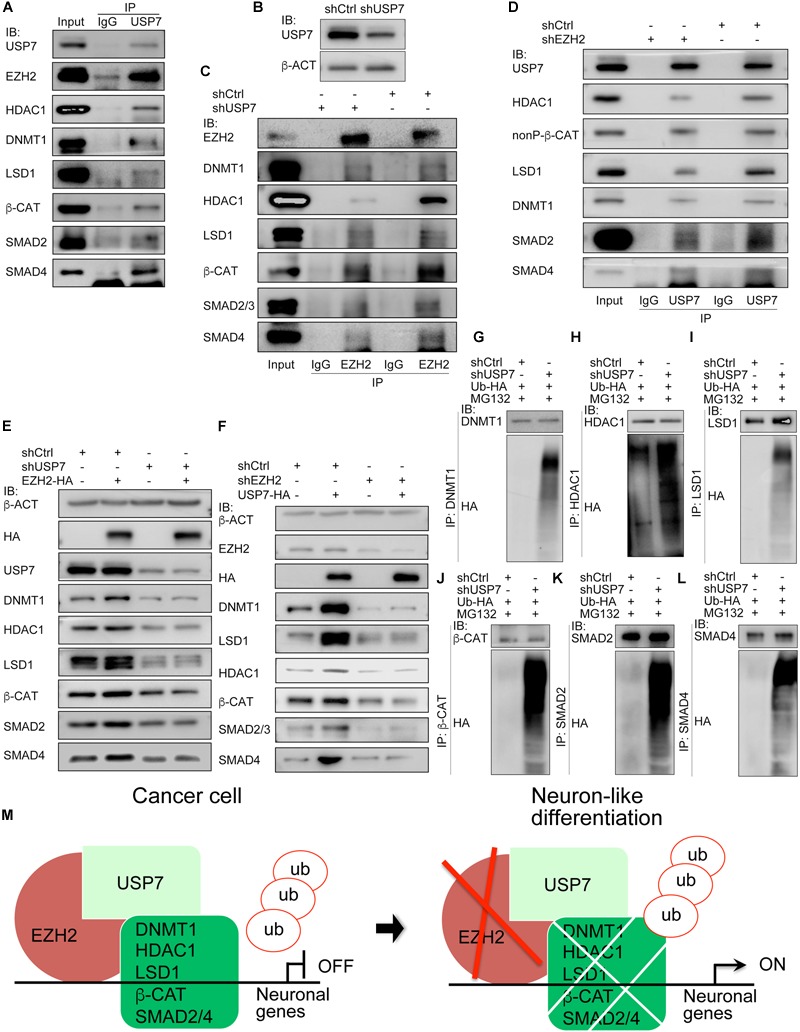
USP7 interacts with EZH2 and is required for EZH2 mediated protein stability. (A) Co-IP detection of the interactions between USP7 and EZH2, and between USP7 and EZH2 interaction partners. Immunoprecipitation with IgG was used as a negative control. (B–D) The mutual dependence of EZH2 and USP7 on each other’s interaction with proteins detected. (B) Detection of knockdown efficiency of a USP7 short-hairpin RNA (shUSP7). β-ACT was used as a loading control for IB assays. (C) Influence of USP7 knockdown on the interactions between EZH2 and other proteins. Proteins were immunoprecicpitated with an EZH2 antibody from cells without and with shUSP7. (D) Effect of EZH2 knockdown on the interactions between USP7 and other proteins. Proteins were immunoprecicpitated with a USP7 antibody from cells without and with shEZH2. In (C,D), immunoprecipitation with IgG was used as a negative control. (E,F) Interdependence of EZH2 and USP7 on their ability to maintain protein level. (E) USP7 knockdown counteracted the enhancement of protein level induced by EZH2 overexpression. (F) Knockdown of EZH2 compromised increment of protein level induced by USP7 overexpression. In (E,F), b-ACT expression was detected as a loading control in IB assays. (G–L) Effect of USP7 knockdown on the ubiquitination of proteins. Proteins were immunoprecipitated with antibodies against DNMT1 (G), HDAC1 (H), LSD1 (I), β-CAT (J), SMAD2 (K), and SMAD4 (L), respectively, from cells co-transfected with shCtrl or shUSP7 and HA-tagged ubiquitin plasmid, and treated with MG132. Precipitated proteins were detected for their ubiquitination levels using an HA antibody. (M) A working model depicting the molecular mechanism by which EZH2 regulates neuron-like differentiation in cancer cells.
