Fig. 1.
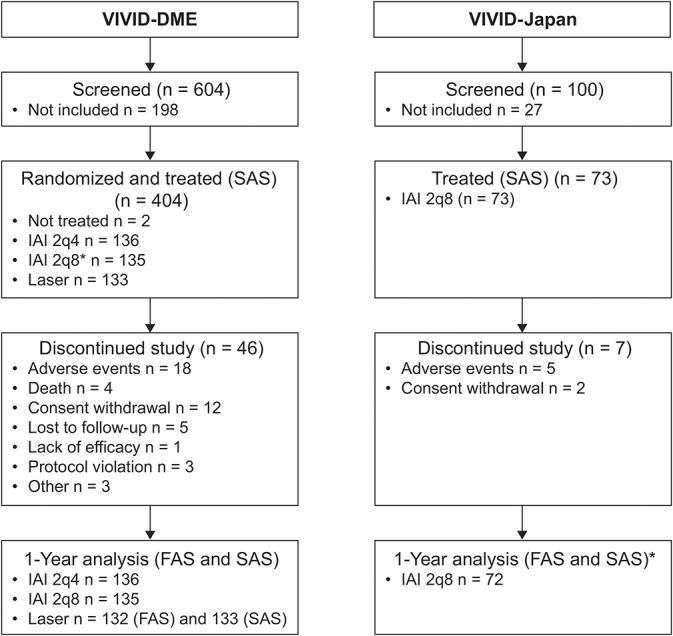
Patient disposition in VIVID-DME and VIVID-Japan. *Of the 73 patients assigned to treatment, one patient withdrew consent and was excluded from the analyses. 2q8, 2 mg every 4 weeks (2q4) from baseline to Week 16 (5 doses) followed by dosing every 8 weeks through Week 48; FAS, full analysis set; SAS, safety analysis set.
