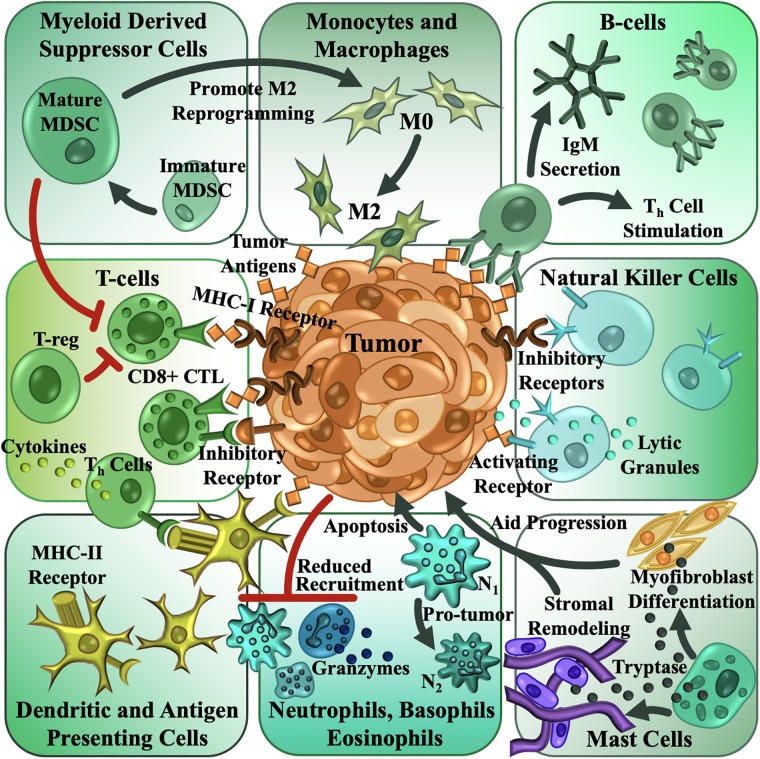
Fig 4. The immune microenvironment of tumors contains cellular components from both the innate and adaptive immune systems, with functional immuno-modulation between all the different cell types.
Macrophages are typically the most abundant population of leukocytes within the TME, derived from both tissue-resident and circulating monocytic progenitors. The accumulation of tumor-associated macrophages is often correlated with the development of pathological phenotypes in cancer, which leads to the promotion of angiogenesis, metastasis, chemoresistance and functional suppression of adaptive immunity. The TME counterbalances activating natural killer (NK) cell signals with strong inhibitory signals to escape NK cell mediated immune surveillance and further reduce the phagocytic activity of NK cells. NK cells also exhibit functional anergic phenotypes with reduced phagocytosis and reduced amounts of cytoplasmic granules that contribute to tumor progression. Other granulocytes within the TME often recruited from circulating vasculature include neutrophils, basophils, eosinophils and mast cells. Tumors often experience reduced recruitment, but granulocytes are often re-programmed to a pro-tumor phenotype, promoting vascular normalization and stromal remodeling. Analysis of several solid tumors also indicate that they are infiltrated with T-cells and B-cells, recruited from circulating blood and lymphatic structures. The number of infiltrated T-cells offer significant prognostic value to cancers. However, the TME reprograms T-cells into an exhausted anergic state, leading to severe immune suppression, specifically of the Th and CTL (CD8+ cytotoxic T lymphocytes) phenotypes. Additionally, recruited naive T-cells are also converted to an insidious regulatory Treg phenotype, which contributes to suppressive immunomodulation. B-cells typically respond to tumor-derived antigens and elicit antibody responses through IgM secretion and direct stimulation of Th cells. Tumor-educated B-cells are immuno-suppressive, promote regulatory T-cells, and promote carcinogenesis. Myeloid derived suppressor cells are heterogeneous mixes of immature myeloid cells, found accumulated in lymphoid structures, blood, and the TME, and are heavily correlated with immune suppression. Myeloid derived suppressor cells are powerful inactivators of T-cells. Impaired myeloid differentiation also results in defective antigen presentation. Coupled with dysregulated T-cell priming by antigen presenters like dendritic cells, an overall immune suppressive landscape leads to tumor escape from immune surveillance.