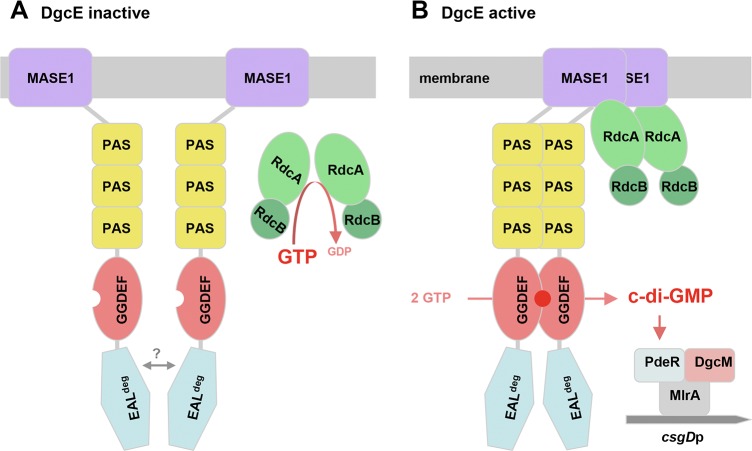
Fig 7. Model of the control of DgcE activity by the GTPase RdcA and its accessory factor RdcB.
A: At high cellular GTP levels, the RdcA/RdcB complex is predominantly in the GTP-bound form that is unable to interact with the MASE1 domain of DgcE, whose GGDEF domain remains in the monomeric and enzymatically inactive form since the C-terminal EAL domain counteracts dimerization via the PAS3 region. B: During entry into stationary phase, the cellular GTP level is decreased substantially, the RdcA/RdcB complex is predominantly in the nucleotide-free form that binds to the MASE1 domains of two DgcE molecules, which promotes an alignment of the PAS3 and GGDEF domains, thereby overcoming the anti-oligomerizing effect of the EAL domain of DgcE. For simplicity, the c-di-GMP production-promoting forms of both the RdcA/RdcB complex as well as DgcE are depicted here as dimers, but these may also form higher order oligomers (as suggested by immunoblot data in Fig 3). C-di-GMP produced by the GGDEF domains is bound and degraded by the trigger PDE PdeR, thereby relieving inhibition imposed by PdeR onto DgcM and the transcription factor MlrA, which results in the initiation of csgD transcription by the DgcM/MlrA complex.