Abstract
Including animals in autism intervention is growing in both research and practice. A systematic literature review was conducted to collate and synthesize all empirical research on animal-assisted intervention (AAI) for autism published from 2012 to 2015. Findings from 28 included studies revealed that AAI programs generally include one animal per participant with a total contact time of approximately 10 hours over the course of 8 to 12 weeks. Research methodology is diverse and though limited in many cases, has improved over the last few years. The most commonly reported outcome was increased social interaction, which was unanimously significant across 22 studies. The need for further research is highlighted, calling for a focus on refining AAI techniques, identifying optimal circumstances for positive change as well as individuals who may not benefit, and independent replication of high quality studies to move AAI from an enrichment activity to an evidence-based practice for autism.
Keywords: animal-assisted intervention, animal-assisted therapy, animal-assisted activities, autism spectrum disorder, children, human-animal interaction, social interaction
There is a growing momentum in research and clinical practice related to the inclusion of animals in a broad range of intervention services, particularly those for autism spectrum disorder (O’Haire, 2013). Integrating animals into therapeutic programming is known as Animal-Assisted Intervention (AAI), and is comprised of three categories: targeted therapeutic services (Animal-Assisted Therapy, AAT), enrichment visits (Animal-Assisted Activities, AAA), and educational programs (Animal-Assisted Education, AAE; Fine, Tedeschi, & Elvolve, 2015). Its provision spans a wide spectrum of populations, from typically-developing children to adults with psychiatric disorders (Barker & Wolen, 2008; O’Haire, 2010). As the clinical practice of AAI for autism increases in prevalence, there is a critical need for scientific evaluation and, if potentially efficacious, the development of evidence-based best practices (Grandin et al., 2015; Palley, O’Rourke, & Niemi, 2010).
Including animals in autism services may stem from the fact that many reported outcomes of interacting with animals map roughly onto challenges characteristically associated with autism, most notably social relationships and stress. Though autism is a spectrum disorder with highly individualized difficulties, social deficits represent the core underlying feature and source of impairment (Carter, Davis, Klin, & Volkmar, 2005). Social challenges can include difficulty engaging in social interactions and forming social relationships (Jobe & White, 2007). The presence of animals has been linked to increased social interaction among communities (e.g. Wood, Giles-Corti, & Bulsara, 2005). It is possible that an animal may act as a social facilitator to connect individuals with autism to the people around them (e.g., Sams, Fortney, & Willenbring, 2006). Animals have been documented to uniquely elicit social interactions, above and beyond other traditional objects of engagement such as toys (O’Haire, McKenzie, Beck, & Slaughter, 2013). The presence of animals has been shown to change people’s perception other humans, rating people with animals as friendlier, happier, and more approachable than those without animals (Rossbach & Wilson, 1992). Individuals with autism may lack opportunities for positive peer interaction (White, Koenig, & Scahill, 2007); thus if animals can provide an appealing motivator for individuals to connect and practice social interactions in a naturalistic environment, then their presence may be conducive to fostering social development in addition to symptom reduction through Animal-Assisted Intervention.
Animals also have an evidenced ability to influence human psychobiology via stress reduction in social situations (e.g., Beetz, Julius, Turner, & Kotrschal, 2012). When faced with social ostracism, people tend to have lower stress levels if an animal is present, compared to a human companion (e.g., Polheber & Matchock, 2013). Children with autism experience heightened social anxiety and are sometimes bullied and rejected by their peers (e.g., Bellini, 2006). The presence of an animal may ameliorate some feelings of social stress by acting as a buffer and positive focus of attention (Fine & Beck, 2015). Recent neurobiological evidence suggests that children with autism may perceive greater social reward from animal faces, compared to human faces, as indicated by greater activation in brain regions related to reward and emotional arousal such as the amygdala and putamen (Whyte, Behrmann, Minshew, Garcia, & Scherf, 2015). Face-to-face interactions with animals may be more appealing and less threatening than those with human conspecifics alone (Solomon, 2012). Though promising, the potential benefits of interacting with animals for autism have been predominantly anecdotal.
Recent systematic reviews have gathered the published, empirical literature on AAI for autism prior to 2014. One review identified 14 studies published between 1989 and 2012 (O’Haire, 2013). The findings were predominantly positive, revealing preliminary proof of concept for AAI for autism; however, the study designs and methodology were notably weak. Another review, focused only on studies with outcomes related solely to core autism diagnostic criteria, identified 20 studies published between 1989 and 2013 (Davis et al., 2015). This group of studies reported mixed and positive findings related to autism symptoms, with several noted threats to internal validity compromising the robustness of the outcomes. Across both reviews, the majority of studies had been published later in the periods covered (between 2010 and 2013), indicating a growing momentum in research on AAI for autism. Neither of the existing reviews examined the most recent studies between 2013 and 2015.
Given the multidisciplinary nature of research on AAI, it is important to periodically collate and capture the latest findings across a broad range of fields of study, including psychology, animal behavior, sociology, nursing, medicine, and others. The overall goal of this systematic literature review is to identify and synthesize all published, empirical research studies which report outcomes of AAI for autism since the last inclusive systematic review, that is, literature published between 2012 and early 2016. The specific aims are to (a) describe the key characteristics of AAI for autism, (b) evaluate the state of the evidence base, and (c) summarize the reported outcomes.
Methods
Protocol and Eligibility
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to perform this review (Liberati et al., 2009; Moher, Liberati, Tetzlaff, & Altman, 2009). The study procedures were defined a priori in a protocol that specified the search strategy, inclusion and exclusion criteria, and data extraction items. Inclusion criteria replicated the O’Haire (2013) systematic review and consisted of (a) publication in English in a peer-reviewed journal, (b) collection of empirical data on AAI or companion animal ownership, where empirical is defined as the systematic collection and reporting of original observational or experimental scientific research, and (c) reporting of outcome results for participants with autism.
Search Procedure
Studies were identified by searching the following electronic databases for articles published from the cutoff date for our last systematic review (June 2012) through January, 2016: ERIC, Campbell Library, ClinicalTrials.gov, Medline, ProQuest, PsycARTICLES, PsycINFO, Scopus, and HABRI Central. Search terms for all databases included (1) at least one identifier for autism spectrum disorder and (2) at least one identifier for AAI or pet ownership in the full text of the article. Identifiers for autism included autism OR autistic OR asperger(s) OR pervasive developmental disorder(s). Identifiers for AAI included a comprehensive list of 38 search terms replicated from a prior systematic review (O’Haire, 2013).
Data Extraction and Evaluation
Information was extracted from each included study to achieve the three aims of the review. To achieve the first aim—describing key characteristics of the AAIs—data items included AAI terminology, species, setting, ratio of interventionists/personnel to participants to animals, animal/handler certifications, and dose (program duration, session frequency, session length, and total contact hours). To achieve the second aim—evaluating study methodology and risk of bias—data items included sample size, sample demographics (age, gender, diagnosis), study design and effect size, control/comparison condition, assessment measures (including type, standardized instruments, and raters/informants), ethical approval to conduct research with humans and animals, and effect size. For the third aim—summarizing outcomes—data items included the measures and results of each study, subsequently organized by the most commonly evaluated outcomes. Additional data items were extracted for study identification and exploratory purposes, including first author, publication year, country of corresponding author, and journal name.
Results
Study Selection
The initial literature search identified 548 articles published between 2012 and 2016. A flowchart of the study exclusion process is presented in Figure 1. The final sample included 28 articles (5.1% of the total initial pool) which met the inclusion criteria of empirically evaluating and reporting outcomes of AAI for autism.
Fig. 1.
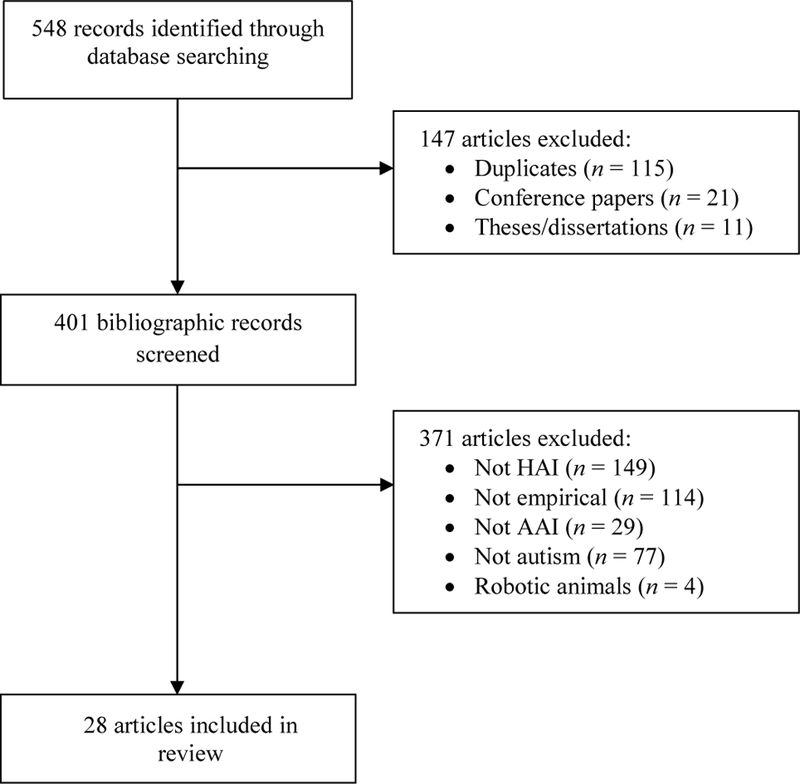
Flow chart of study selection process. HAI human animal interaction, AAI animal-assisted intervention.
The number of studies increased each year, with 4 in 2012, 5 in 2013, 9 in 2014, and 10 in 2015. Though all included articles were published in English, the corresponding authors were spread around the globe. Approximately 36% were from the United States (10 studies), with the remainder from Australia, Hong Kong, Spain, the United Kingdom (2 studies each), and France, Hungary, Iran, Ireland, Italy, Japan, Portugal, Romania, Singapore, and Sweden (1 study each). The journal sources represented a broad range of disciplines, including autism (9 studies), medicine (5 studies), education (4 studies), psychology (4 studies), human-animal interaction and multidisciplinary (2 studies each), and neurochemistry and nursing (1 study each). The study designs, samples, and outcome measures were diverse; thus the results of this review will focus on descriptive and qualitative synthesis rather than meta-analysis.
Characteristics of AAI for autism
To describe the key characteristics of AAI for autism (aim 1), several features of AAI in each of 22 included studies were summarized (see Table 1). Information on companion animals (n = 5) and service animals (n = 1) was not included in the characteristics synthesis given that the format is qualitatively different.
Table 1:
List of terms to identify animal-assisted intervention (AAI) in database search
| Animal intervention | Canine therapy | Dolphin assisted | Human animal | Therapeutic animal(s) |
| Animal therapy | Canine assisted | Dolphin facilitated | interaction(s) | Therapeutic dog(s) |
| Animal assisted | Canine facilitated | Equine therapy | Pet therapy | Therapeutic horse(s) |
| Animal facilitated | Companion animal(s) | Equine assisted | Pet assisted | Therapeutic horseback |
| Anthrozoology | Dog therapy | Equine facilitated | Pet facilitated | Therapeutic pet(s) |
| Assistance animal(s) | Dog assisted | Hippotherapy | Service animal(s) | Therapeutic riding |
| Assistance dog(s) | Dog facilitated | Horseback riding | Service dog(s) | Therapy with animals |
| Assistance horse(s) | Dolphin therapy | Human animal bond | Service horse(s) |
Note: Bold terms were those used in the final review sample.
Terminology.
The terminology used to denote AAI included 14 terms across 22 studies. Half (n = 11) used a variant of the field-recommended terms with the word “assisted”, such as “animal-assisted activities” or “equine-assisted activities.” The next most common terms included “therapeutic horseback riding” (n = 7) and “hippotherapy” (n = 2).
Species.
The most common animals in AAI were horses (n = 12), which accounted for 55% of the studies, followed by dogs (n = 5), guinea pigs (n = 3), and dolphins (n = 2). No studies of AAI included multiple species, thus no direct comparisons between species were made. Almost half of the studies (n = 10) did not report information about animal/handler certification or registration. Of those that did (n = 12), the most common certification was through the Professional Association of Therapeutic Horsemanship (PATH) International (n = 6).
Ratios.
To determine the personnel allocation for AAI, the ratio of personnel to participants was compiled. In some cases, the horse studies indicated following PATH standards but did not report any volunteers present in the sessions. The following numbers were calculated with the reported individuals present, rather than making assumptions on personnel not reported. The average number of personnel per participant was 1.7, including individuals with a range of expertise and experience such as therapists, riding instructors, animal handlers, and volunteers. The average was higher in AAI with horses (2.3) than with other species (1.0). As a rough indication of the animal experience, we assessed the ratio of participants to animals. The average number of participants per animal was 1.7, though most studies had one or fewer participants per animal (82%, n = 18).
Dose.
Dose was evaluated for each AAI by extracting the total program duration, number of sessions, and session length. These data were then used to calculate the session frequency and total contact time over the duration of the program. AAI programs ranged in duration from 1 to 52 weeks. The average program duration was 12.8 weeks (SD = 11.5), but most programs lasted between 8 to 12 weeks (55%, n = 12). The frequency of sessions ranged from 0.1 to 4.6 sessions per week, at 1.4 on average (SD = 0.8). Session length ranged from 1 to 75 minutes, with most lasting between 15 to 60 minutes (86%, n = 19); average session length was 34.7 minutes (SD = 18.9). The total contact time, indicating time spent with animals, ranged from 1 minute to 65 hours, at 10.1 hours on average (SD = 13.9) over the course of the entire program. Programs with horses were slightly shorter (ΔM = −3.5 weeks) and less frequent (ΔM = −0.4 sessions per week) than programs with other species; however the total contact time was roughly the same (ΔM = −0.2 total contact hours) due to longer sessions (ΔM = 16.0 minutes per session) with horses than other species.
Methodology and Risk of Bias
To evaluate study methodology and risk of bias (aim 1), selected elements of each study’s methods were extracted. The sample size, study design, assessment type, and raters/informants are presented in Table 2 and the comparison condition is presented along with outcomes in Table 3 for all 28 studies reviewed.
Table 2:
Overview of animal-assisted intervention (AAI) characteristics, categorized by species and sorted by sample size
| First Author (Year) | N | Terminology | Setting | Ratio (Personnel: Participants: Animals) |
Animal/Handler Certification |
Dose |
|||
|---|---|---|---|---|---|---|---|---|---|
| Program duration (weeks) |
Session frequency (per week) | Session length (minutes) |
Total contact time (hours) | ||||||
| HORSES | |||||||||
| Gabriels (2015) | 116 | Therapeutic horseback riding | Riding center | 2:1:1 | PATH | 10 | 1 | 45 | 7.5 |
| Borgi (2015) | 28 | Equine-assisted therapy | Riding center | 2:3:3 | FISE | 25 | 1 | 65 | 27.1 |
| Steiner (2015) | 26 | Therapeutic horse riding | Riding center | 4:1:1 | - | 4 | 1 | 30 | 2.0 |
| Lanning (2014) | 25 | Equine-assisted activities | Riding center | 4:1:1 | PATH | 12 | 1 | 60 | 12.0 |
| Ward (2013) | 21 | Therapeutic horseback riding | Riding center | 4:1:1 | PATH | 30 | 1 | 60 | 18.0 |
| Garcia-Gomez (2014) | 16 | Therapeutic horse riding | Riding center | 1:4:4 | PATH | 12 | 2 | 45 | 18.0 |
| Tabares (2012) | 8 | Hippotherapy | Riding center | 1:1:1 | AZE | 4 | 1 | 30 | 2.0 |
| Ajzenman (2013) | 7 | Hippotherapy | Riding center | 1:3:3 | PATH | 12 | 1 | 45 | 9.0 |
| Jenkins (2013) | 7 | Therapeutic horseback riding | Riding center | 4:1:1 | PATH | 9 | 1 | 40 | 6.0 |
| Ghorban (2013) | 6 | Therapeutic horseback riding | Riding center | 3:2:2 | - | 4 | 2 | 45 | 6.0 |
| Chen (2015) | 4 | Equine-assisted activity | Riding center | -:1:1 | - | 1 | 1 | 1 | 0.0 |
| Holm (2014) | 3 | Therapeutic horseback riding | Riding center | 4:1:1 | PACTH, NARHA | 12 | 2 | 30–45 | 12.5 |
| DOGS | |||||||||
| Fung (2014) | 10 | Therapy dog | School | 1:1:2 | ASA | 7 | 3 | 20 | 6.7 |
| Grigore (2014) | 3 | Animal-assisted therapy | Treatment center | 2:1:1 | RAAATA | 10 | 3 | 15 | 2.5 |
| Stevenson (2015) | 3 | Sessions with a dog | School | 2:1:1 | - | 10 | 1 | 20 | 1.7 |
| Funahashi (2014) | 2 | Animal-assisted activities | Laboratory | 1:1:3 | - | 28 | <1 | 35 | 2.3 |
| Fung (2015) | 1 | Animal-assisted play therapy | School | 1:1:1 | ASA | 5 | 3 | 20 | 4.7 |
| GUINEA PIGS | |||||||||
| O’Haire (2015) | 99 | Animal-assisted activities | School | 1:3:2 | - | 8 | 2 | 20 | 5.3 |
| O’Haire (2014) | 64 | Animal-assisted activities | School | 1:3:2 | - | 8 | 2 | 20 | 5.3 |
| O’Haire (2013) | 33 | Animal-assisted activities | School | 1:3:2 | - | 8 | 2 | 20 | 5.3 |
| DOLPHINS | |||||||||
| MdYusof (2012) | 15 | Dolphin-assisted therapy | Dolphin center | 3:15:1 | - | 52 | 1 | 75 | 65.0 |
| Salgueiro (2012) | 10 | Dolphin interaction | Dolphin center | 2:1:1 | - | 12 | 1 | 15 | 3.0 |
Notes: Information is reported for the animal-assisted intervention (AAI) condition only, not any control/comparison conditions. Session frequency is the average number of sessions per week, rounded to the nearest whole number. - = not reported, ASA = Animal Asia Foundation, RAAATA = Romanian Association of Animal-Assisted Therapy and Activities, PATH = Professional Association of Therapeutic Horsemanship, FISE = Italian Equestrian Federation, PACTH = Pennsylvania Council on Therapeutic Horsemanship, NARHA = North American Riding for the Handicapped Association, AZE = Association of Zootherapy of Extremadura
Table 3:
Research study design and assessment types, categorized by species and sorted by sample size
| First author (Year) | N | Study design | Assessment Type | Raters/Informants | Ethics Review | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Survey | Interview | Observation | Physiology | Research team | Parent | Teacher | Self | Blinded | Human | Animal | ||||||
| HORSES | |||||||||||||||||
| Gabriels (2015) | 116 | Randomized control | x | x | - | x | - | x | x | - | - | x | x | - | |||
| Borgi (2015) | 28 | Randomized control | - | x | - | x | - | x | x | - | - | x | x | - | |||
| Steiner (2015) | 26 | Randomized control (ABA) | - | x | - | - | x | x | - | - | - | - | x | - | |||
| Lanning (2014) | 25 | Non-randomized control | - | x | - | - | - | - | x | - | x | - | x | - | |||
| Ward (2013) | 21 | Repeated measures (ABAB) | - | x | - | - | - | - | x | - | - | - | x | - | |||
| Garcia-Gomez (2014) | 16 | Non-randomized control | x | x | x | - | - | - | x | x | - | - | - | - | |||
| Tabares (2012) | 8 | Pre-post | - | - | - | - | x | - | - | - | - | - | x | - | |||
| Ajzenman (2013) | 7 | Pre-post | x | x | - | x | - | x | x | - | - | - | x | - | |||
| Jenkins (2013) | 7 | Single-subject (AB) | - | x | - | x | - | x | x | x | - | - | - | - | |||
| Ghorban (2013) | 6 | Pre-post | - | x | - | - | - | - | x | - | - | - | - | - | |||
| Chen (2015) | 4 | Single-subject (AB) | - | - | - | - | x | - | - | - | - | - | x | x | |||
| Holm (2014) | 3 | Single-subject (ABA) | - | - | - | x | - | x | x | - | - | - | x | - | |||
| DOGS | |||||||||||||||||
| Fung (2014) | 10 | Randomized control | x | - | - | x | - | x | - | - | - | x | x | x | |||
| Grigore (2014) | 3 | Single-subject (ABAC) | x | - | - | x | - | x | - | - | - | - | x | - | |||
| Stevenson (2015) | 3 | Single-subject (A) | - | x | - | x | - | x | - | x | - | x | - | - | |||
| Funahashi (2014) | 2 | Single-subject (A) | - | - | - | x | x | x | - | - | - | - | x | - | |||
| Fung (2015) | 1 | Single-subject (ABA) | - | - | - | x | - | x | - | - | - | x | - | - | |||
| GUINEA PIGS | |||||||||||||||||
| O’Haire (2015) | 99 | Repeated measures (AB) | x | x | - | - | x | - | - | - | x | - | x | x | |||
| O’Haire (2014) | 64 | Repeated measures (AB) | x | x | - | - | - | - | x | x | - | - | x | x | |||
| O’Haire (2013) | 33 | Repeated measures (ABA) | x | - | - | x | - | x | - | - | - | x | x | x | |||
| DOLPHINS | |||||||||||||||||
| MdYusof (2012) | 15 | Pre-post | x | x | - | - | - | - | x | - | - | - | - | - | |||
| Salgueiro (2012) | 10 | Pre-post-follow-up | - | x | - | x | - | x | x | - | - | - | x | x | |||
| COMPANION ANIMALS | |||||||||||||||||
| Carlisle (2014) | 70 | Qualitative | - | - | x | - | - | - | x | - | - | - | x | - | |||
| Carlisle (2015) | 70 | Non-randomized control | - | x | - | - | - | - | x | - | x | - | x | - | |||
| Wright (2015) | 62 | Non-randomized control | x | x | - | - | - | - | x | - | - | - | x | - | |||
| Grandgeorge (2012) | 40 | Non-randomized control | - | - | x | - | - | - | x | - | - | - | x | - | |||
| Bystrom (2015) | 13 | Qualitative | - | - | x | - | - | - | x | - | - | - | x | - | |||
| SERVICE ANIMALS | |||||||||||||||||
| Burgoyne (2014) | 164 | Non-randomized control | - | x | - | - | - | - | x | - | x | - | x | - | |||
Sample.
Sample sizes ranged from 1 to 164 participants. Approximately half of the studies (54%, n = 15) had relatively small sample sizes of ≤ 20 and a quarter (25%, n = 7) had larger sample sizes of over 60 participants. All studies focused on children and/or adolescents, with no studies of adults. Participants ranged in age from 2 to 20 years. The mean age across studies was 10.5 years, based on 23 studies which provided the mean or enough information to calculate it. Gender was biased towards males, with 79% male (733 of 926 participants).
Design and Effect Size.
Most studies (n = 26 of 28) used quantitative (rather than qualitative) designs: approximately 62% (n = 16) were within-participants, while the other 38% (n = 10) were between participants. The within-participants studies consisted of single-subject designs (n = 7), simple pre-post (n = 5), and repeated measures with control conditions (n = 4). The between-participants studies consisted of non-randomized (n = 6) and randomized (n = 4) control designs. Only 10 of 28 studies (36%) reported an effect size metric, which was most commonly Cohen’s d (n = 7).
Control/comparison.
Nearly a third (32%) of studies either did not have a control condition (n = 8) or did not report the content of the control condition (n = 1). Half of the remaining studies included an active control or placebo, which consisted of either sessions without an animal present (n = 5) or sessions with an alternative focus of attention through toys (n = 5). The other half included a no-treatment control, either via a waitlist period (n = 3), withdrawal period (n = 2), or in the case of companion animal studies, homes without pets (n = 4). Two studies also included a comparison group of typically-developing children who experienced the same experimental conditions as children with autism.
Assessment.
The majority of studies (55%, n = 21) used a survey or interview assessment technique. Other types of assessments included behavioral observation (n = 12) and physiological assessment (n = 5). All but five studies (82%, n = 23) incorporated at least one standardized assessment tool, rather than using an investigator-designed instrument alone. Raters or informants included parents (n = 17), research staff (n = 13), teachers (n = 5), and children with autism themselves (n = 4). For studies with behavioral observation, half (50%, n = 6) reported using blinded observers to reduce the risk of bias. Physiological assessments included electrodermal activity (EDA), electroencephalogram (EEG), electromyography (EMG), gait cycle analysis, salivary cortisol and salivary progesterone, in one study each. Most studies (64%, n = 18) used only one assessment technique, rather than multi-modal assessments.
Ethical Review.
Most studies reported on an Institutional Review Board (IRB) or equivalent approval for conducting research with human participants (79%, n = 22). Only 6 studies reported on an Institutional Animal Care and Use Committee (IACUC) or equivalent approval for conducting research with animals (21%).
Outcomes of AAI for autism
To summarize reported outcomes (aim 3), key findings were categorized by the frequency of their reporting across the 28 included studies (Table 4). Given the potential risk of bias identified in the methodological review, findings should be interpreted as preliminary in most cases.
Table 4:
Comparison conditions, measures, and outcomes, categorized by species and sorted by sample size
| First Author (Year) |
N | Control/Comparison Condition | Standardized Outcome Measures | Outcomes |
|---|---|---|---|---|
|
HORSES |
||||
| Gabriels (2015) | 116 | Toy horse | ABC SRS SALT VABS/BOT/PPVT |
△ Self regulation: irritability, hyperactivity (control*) — Self regulation: lethargy, stereotypy, inappropriate speech (control – ) ▲ Social: communication (control**), cognition (control*) — Social: awareness, motivation, autistic mannerisms (control – ) ▲ Communication: different words, new words (control**) — Adaptive behaviors, motor skills, receptive vocabulary (control – ) |
| Borgi (2015) | 28 | Waitlist | VABS TOL |
▲ Adaptive behaviors: socialization, motor skills (pre*, control*) Adaptive behaviors: communication, daily living skills (pre*, control – ) ▲ Executive function: planning time (pre*, control*) Executive function: execution time, accuracy (pre*, control – ) |
| Steiner (2015) | 26 | Physical therapy, Withdrawal | APAS PAC |
▲ Gait cycle and balance (pre*, control*) ▲ Education: communication, self-care, motor skills, socialization (pre***, control***) |
| Lanning (2014) | 25 | Social circles | PedsQL CHQ |
△Quality of life functioning: physical, emotional, social (pre*, control – ) — Health: physical, psychosocial (pre –, control – ) |
| Ward (2013) | 21 | Withdrawal | GARS SPSC |
▲ Autism diagnostic: overall, social interaction (pre*) — Autism diagnostic: communication, stereotypy (pre – ) ▲ Sensory processing: registration, sensitivity, auditory, visual, touch (pre*) — Sensory processing: movement (pre – ) |
| García-Gómez (2014) | 16 | Not reported | BASC - |
▲ Behavior: aggressiveness (pre*), hyperactivity, atypicality (pre†) — Behavior: attention, learning, depression, anxiety, withdrawal, somatization, externalizing, internalizing, school, social, leadership, study, adaptive (pre – ) ▲ Quality of life: interpersonal relations, social inclusion (control*), overall (control†) — Quality of life: emotion, physical, family, personal, self-determination (control – ) |
| Tabares (2012) | 8 | None | ELISA | ▲ Salivary cortisol (pre*) ▲ Salivary progesterone (pre*) |
| Ajzenman (2013) | 7 | None | VABS CACS - |
▲ Adaptive behaviors: overall, communication, socialization (pre*), daily living skills (pre†) — Adaptive behaviors: motor skills (pre – ) ▲ Age-appropriate: social interaction, self-care, low-demand leisure (pre*) — Age-appropriate: community mobility, high-demand leisure, domestic, education (pre – ) ▲ Motor control and postural stability (pre*) |
| Jenkins (2013) | 7 | After-school program, Waitlist |
CBCL - |
— Problem behaviors: overall, internalizing, externalizing (pre –, control – ) — Behaviors: affect, language, off-task, compliance, problem behaviors (pre –, control – ) ▲ Posture (pre) |
| Ghorban (2013) | 6 | None | TSSA | ▲ Social skills: overall, affective understanding, perspective taking, initiating (pre*) and maintaining interaction (pre**) — Social skills: responding to interaction (pre – ) |
| Chen (2015) | 4 | Toy, TD child | EEG | ▲ Right frontal lobe activity/asymmetry (control, TD) |
| Holm (2014) | 3 | Withdrawal | ABC SRS - |
▲ Self regulation: lethargy, stereotypy (pre) Self regulation: irritability, hyperactivity (pre) for 2 of 3 participants — Self regulation: inappropriate speech (pre – ) ▲ Social: awareness, cognition, communication, motivation, autistic mannerisms (pre) ▲ Spontaneous verbalization, physical stereotypy (pre) |
|
DOGS |
||||
| Fung (2014) | 10 | Doll | - | △ Social and non-social behaviors (pre*, control – ) |
| Stevenson (2015) | 3 | Partial session without animal | ADOS - |
— Autism diagnostic criteria (pre, control) ▲ Social behaviors, isolation (pre, control) △ Shared enjoyment (pre, control) for 2 of 3 participants |
| Grigore (2014) | 3 | Social story without animal | - | ▲ Initiation of social interaction (pre, control) △ Appropriate social interactions, level of prompting (pre, control) for 2 of 3 participants |
| Funahashi (2014) | 2 | None | EMG - |
▲ Smiling ▲ Positive and negative social behaviors |
| Fung (2015) | 1 | Sessions without animal | - | ▲ Social and non-social behaviors (pre, control) |
|
GUINEA PIGS |
||||
| O’Haire (2015) | 99 | Toys, TD children |
EDA - |
▲ Arousal: skin conductance responses (control**, TD**), level (control*, TD*) ▲ Positive emotions (control***, TD – ) |
| O’Haire (2014) | 64 | Waitlist | PDDBI SSRS |
▲ Social approach/withdrawal behaviors (pre*, control*) △ Social skills (pre*, control**) — Problem behaviors (pre –, control – ) |
| O’Haire (2013) | 33 | Toys | OHAIRE | ▲ Social interaction, communication, isolation, positive emotions (control***) — Problem behaviors (control – ) |
|
DOLPHINS |
||||
| MdYusof (2012) | 15 | None | GARS | ▲ Autism diagnostic: overall, social interaction, communication, stereotypy (pre***) |
| Salgueiro (2012) | 10 | None | PEP-R CARS/ATEC/- - |
▲ Development: overall, cognitive verbal (pre**), fine motor, cognitive performance (pre*) — Development: imitation, perception, gross motor, eye-hand integration (pre – ) — Autism diagnostic, treatment outcomes, theory of mind (pre – ) ▲ Social behavioral complexity (pre***) |
|
COMPANION ANIMALS |
||||
| Carlisle (2014) | 70 | No pet | - | ▲ Responsibility, companionship, happiness, love, social interaction, empathy, safety, stress |
| Carlisle (2015) | 70 | No pet | SSIS | Social skills (control†) — Problem behaviors (control – ) |
| Wright (2015) | 62 | No pet | PSI | ▲ Parenting stress: overall, distress (pre**, control*), difficulty of child (pre**, control**) — Parenting stress: parent-child dysfunctional interaction (pre –, control – ) |
| Grandgeorge (2012) | 40 | No pet | ADI-R | ▲ Autism diagnostic: prosocial behaviors (control***) — Autism diagnostic: social interactions, communication, repetitive behaviors (control – ) |
| Bystrom (2015) | 13 | None | - | ▲ Interaction with other people, comfort, functioning and development (pre) |
|
ASSISTANCE ANIMALS |
||||
| Burgoyne (2014) | 164 | Waitlist | PCS CGSQ - |
▲ Caregiver competence (control*) — Caregiver strain (control – ) ▲ Safety from environmental hazards, public acceptance (control***) |
Notes: ▲ significant improvement, ρ significant improvement on some comparisons, — non-significant or no change,
p ≤ .10,
p < .05;
p < .01;
p < .001, - = Non-standardized measure, ABC = Aberrant Behavior Checklist, ADI-R = Autism Diagnostic Interview – Revised, ADOS = Autism Diagnostic Observation Schedule, APAS = Ariel Performance Analysis System, ATEC = Autism Treatment Evaluation Checklist, BASC = Behavior Assessment System for Children, BOT = Bruininks-Oseretsky Test of Motor Proficiency, CACS = Child Activity Card Sort, CARS = Childhood Autism Rating Scale, CBCL = Child Behavior Checklist, CGSQ = Caregiver Strain Questionnaire, CHQ = Child Health Questionnaire, EDA = Electrodermal Activity , EEG = Electroencephalogram, ELISA = Enzyme-linked Immunosorbent Assay, EMG = Electromyography, GARS = Gilliam Autism Rating Scale, OHAIRE = Observation of Human-Animal Interaction for Research, PAC = Pedagogical Analysis and Curriculum, PCS = Perceived Competence Scales, PDDBI = Pervasive Developmental Disorder Behavior Inventory, PedsQL = Pediatric Quality of Life Inventory, PEP-R = Psychoeducational Profile – Revised, PPVT = Peabody Picture Vocabulary Test, PSI = Parenting Stress Index, PVQ = Pediatric Volitional Questionnaire, QLES-Q = Quality of Life Enjoyment and Satisfaction – Questionnaire, SALT = Systematic Analysis of Language Transcripts, SIPT = Sensory Integration and Praxis Test, SP = Sensory Profile, SPSC = Sensory Profile School Companion, SRS = Social Responsiveness Scale, SSIS = Social Skills Improvement Rating Scale, SSRS = Social Skills Rating System, TOL = Tower of London, TPCIS = Timberlawn Parent-Child Interaction Scale, TSSA = Triad Social Skills Assessment, VABS = Vineland Adaptive Behavior Scales.
Social interaction.
The most commonly assessed outcome was social interaction, evaluated in 79% (n = 22) of all included studies; all reported positive effects of AAI on social interaction. Changes included increases in social interaction on the Gillian Autism Rating Scale (GARS, n = 2; MdYusof & Chia, 2012; Ward et al, 2013), Pervasive Developmental Disorder Behavior Inventory (PDDBI, n = 1; O’Haire, McKenzie, McCune, & Slaughter, 2014) and Child Activity Card Sort (CACS, n = 1; Ajzenman, Standeven, & Shurtleff, 2013); social skills on the Triad Social Skills Assessment (TSSA, n = 1; Ghorban, Sedigheh, Marzieh, & Yaghoob 2013), Social Skills Rating System (SSRS, n = 1; O’Haire et al., 2014) and its updated version the Social Skills Improvement System (SSIS, n = 1; Carlisle, 2015); socialization on the Vineland Adaptive Behavior Scales (VABS, n = 2; Ajzenman et al., 2013; Borgi, et al., 2015) and Pedagogical Analysis and Curriculum (PAC, n = 1; Steiner & Kertesz, 2015); social responsiveness on the Social Responsiveness Scale (SRS, n = 2; Gabriels, et al., 2015; Holm, et al., 2014), social quality of life on the Pediatric Quality of Life (PedsQL, n = 1; Lanning, et al., 2014) and an investigator designed survey (n = 1; García-Gómez et al., 2014); as well as increased social interaction in qualitative reports (n = 2; Byström & Persson, 2015; Carlisle, 2014) and behavioral observation (n = 8; Fung & Leung, 2014; Fung, 2015; Grigore & Rusu, 2014; Funahashi et al., 2014; Holm et al., 2014; O’Haire et al., 2013; Salgueiro et al., 2012; Stevenson, Jarred, Hinchcliffe, & Roberts, 2015). Nuanced findings included one study showing changes in social communication and cognition, but not motivation and awareness on the SRS following AAI with horses compared to a barn activity control condition (Gabriels, Zhaoxing, et al., 2015); another showed increases in prosocial behaviors but not social interactions on the Autism Diagnostic Interview – Revised (ADI-R) following the introduction of a companion animal in the home (Grandgeorge et al., 2012a). Given the high frequency of positive outcomes related to social interaction, it appears that this is the primary research outcome of AAI for autism. All other categories of findings were less commonly assessed than social interaction.
Language and communication.
Language and communication were evaluated in 43% (n = 12) of included studies. Among these, 75% (n = 9) reported significant improvements, while 25% (n = 3) did not. Significant changes included increases in communication on the VABS (n = 2; Borgi et al., 2015; Ajzenman et al., 2013), PAC (n = 1; Steiner & Kertesz, 2015), Systematic Analysis of Language Transcripts (SALT, n = 1; Gabriels et al., 2015), Psychoeducational Profile Revised (PEP-R, n = 1; Salgueiro et al., 2012), and behavioral observation (n = 3; Holm et al., 2014; O’Haire et al., 2013; Stevenson et al., 2015). Changes were seen in one study using the GARS with dolphins (MdYusof & Chia, 2012), but not on another using the same measure with horses (Ward et al., 2013). No significant changes were reported for communication on the ADI-R for pet ownership (Grandgeorge et al., 2012), nor in a single-subject behavioral observation study of AAI with horses (Jenkins & Reed, 2013). Taken together, it appears that in some, but not all cases, verbal language communication may increase from AAI.
Problem behaviors.
A subset of 29% (n = 8) of studies evaluated problem behaviors and reported mixed findings. Half found no changes in problem behaviors, including on the SSRS (n = 1; O’Haire et al., 2014), SSIS (n = 1; Carlisle, 2015), Child Behavior Checklist (CBCL, n = 1; Jenkins & Reed, 2013), and behavior observation (n = 1; O’Haire et al., 2013). The other studies found some evidence of reduced problem behaviors, including reduced hyperactivity on the ABC (n = 2; Gabriels et al., 2015; Holm et al., 2014) and behavior observation (n = 1; Funahashi et al., 2014). One study showed reduced aggressiveness on the Behavior Assessment System for Children (BASC; García-Gómez et al., 2014), but not reduced internalizing or externalizing behaviors, which comprise the core problem behaviors on the SSRS and SSIS. These finding suggest that internalizing and externalizing problems are likely not affected by AAI, whereas hyperactivity may be modified in some cases.
Positive emotions.
Emotional display and experience were evaluated in 25% (n = 7) of included studies. All reported positive changes in emotional experience from AAI, including increased signals of positive emotion such as smiling assessed via behavioral observation (n = 2; O’Haire et al., 2013; Stevenson et al., 2015) or auto-detection through electromyography (EMG, n = 1; Funahashi et al., 2014), reduced irritability on the ABC (n = 2; García-Gómez et al., 2014; Holm et al., 2014), and qualitative reports by parents (n = 1; Carlisle, 2014) or the child with autism (n = 1; O’Haire, McKenzie, Beck, & Slaughter, 2015). Results from theses seven studies indicate that AAI is related to positive emotional experiences.
Motor Skills.
Motor control and posture were evaluated in 21% (n = 6) of studies. The species in these studies were only horses (n = 5) and dolphins (n = 1). Significant changes were reported in most (n = 5) studies, with some nuances about the type of motor control. Changes included increases in observation of postural stability (n = 2; Ajzenman et al., 2013; Jenkins & Reed, 2013) and fine (but not gross) motor skills (n = 1; Salgueiro et al., 2012) and improvement on physical assessment of gait cycle and balance (n = 1; Steiner & Kertesz, 2015). Standardized assessments yielded mixed results with positive changes in one (Borgi et al., 2015), but not another (Ajzenman et al., 2013), study using the VABS subscale for motor skills, and no changes on the Bruininks-Oseretsky Test of Motor Proficiency (BOT, n = 1; Gabriels et al., 2015). The breadth of measures and differential findings indicate that there is insufficient data to draw conclusions regarding motor skills from AAI at this stage.
Restricted and repetitive behaviors.
Approximately 18% (n = 5) of studies evaluated repetitive behaviors. Most (n = 3) did not find significant changes in this domain and there were contradictory findings for replicated measures. For both the ABC and the GARS, one study found significant changes (ABC: Holm et al., 2014; GARS: MdYusof & Chia, 2012) and another did not (ABC: Gabriels et al., 2015; GARS: Ward et al., 2013). No significant changes were found in the study using the ADI-R domain for restricted/repetitive behavior (Grandgeorge et al., 2012). Thus the findings on stereotypy and restricted/repetitive behaviors are mixed, with weight towards no changes from AAI.
Autism diagnostic evaluation.
Four studies (14%) conducted standardized assessments for autism diagnosis as outcome measures. The findings were split, with half showing significant changes on the GARS (n = 2; MdYusof & Chia, 2012; Ward et al., 2013), and the other half not showing significant changes on the ADOS (n = 1; Stevenson et al., 2015) and on the CARS (n = 1; Salgueiro et al., 2012). Though small, this evidence suggests that AAI should not be considered a stand-alone treatment for autism in its current state.
Stress.
Three studies (11%) assessed and demonstrated reductions in stress or anxious arousal. Measures included salivary cortisol (n = 1; Tabares et al., 2012), electrodermal activity (EDA, n = 1; O’Haire et al., 2015), and qualitative report (n = 1; Carlisle, 2014). The evidence base in this category is notably small, yet cohesive, with respect to individuals with autism. However, two studies (7%) evaluated stress outcomes for parents. Findings were mixed, with one study showing reductions in parenting stress on the Parenting Stress Index (PSI) from companion animals (Wright et al., 2015), and another showing no significant changes in caregiver strain on the Caregiver Strain Questionnaire (CGSQ) from service animals (Burgoyne et al., 2014). Differential findings on stress for the child versus the parent highlight the potential specificity of the target participant of AAI.
Discussion and suggestions for future research
The practice and study of AAI for autism are increasing. In just four years since the last systematic review on the topic (O’Haire, 2013), the empirical literature has tripled, from 14 studies in 2012 to 42 studies in 2015. With this rapidly changing landscape, it is important to collate and synthesize the evidence across the broad range of academic disciplines contributing to the science behind AAI for autism. As noted, the three aims of this systematic review were to synthesize the key intervention characteristics, assess the quality of the research and provide targeted recommendations for ongoing study, and collate the most commonly assessed outcomes of AAI for autism. Here the findings are reviewed by aim, with specific suggestions for future research.
Characteristics of AAI for Autism
In the last systematic review of AAI for autism, only one study used a standardized term for AAI (O’Haire, 2013). Over the last four years, multiple studies have begun to use the recommended terminology of AAI as the umbrella category with its associated subcategories of Animal-Assisted Therapy (AAT) and Animal-Assisted Activities (AAA; Fine et al., 2015). The notable exception is studies with horses, where the term Therapeutic Horseback Riding (THR) appears to be preferred. Despite nuances across some studies, the field of research is unifying towards a standard nomenclature, which is essential to develop evidence-based practices.
The most commonly researched species in AAI is the horse, whereas the most commonly researched species as a companion or service animal is the dog; however, the prevalence of these species in clinical practice may differ. The certification or credentials of the animal and handler were reported in approximately half of the studies. The format and characteristics of each AAI appeared to depend primarily on species. By necessity, AAI with horses took place in riding centers, whereas AAI with other species occurred predominantly in schools. The number of personnel present was higher in AAI with horses, where the personnel: participant ratio was 2:1, compared to 1:1 with other species. Most programs had a 1:1 ratio of participants to animals, which is an important criteria to reduce potential animal welfare problems caused by higher ratios of participants to animals. One strategy to assess attention to animal welfare in AAI research is to identify whether approval has been obtained from an Institutional Animal Care and Use Committee (IACUC) or equivalent for the inclusion of animals in research (Ng et al., 2016). Among the reviewed studies, only 6 of 28 reported such approvals. None specifically targeted and evaluated animal welfare outcomes. Where it is not feasible to conduct statistically powered research on animal welfare concurrent to human outcomes, studies should at a minimum prepare protocols and obtain approval for the inclusion of animals in research with humans through an IACUC or equivalent. For cross-sectional studies that do not involve any animal contact, exemption from these approvals and a supporting explanation should be reported. Yet beyond ensuring the safety of animals in human-focused AAI research, there is a critical need for studies specifically designed to evaluate best practices and develop strategies to enhance animal welfare in clinical practice.
In the included studies, dosing of AAI was highly variable. The total duration of most programs was between 8 to 12 weeks with approximately 1 to 2 sessions per week. Most sessions ranged from 15 to 60 minutes; however, on average, the length of sessions was longer for programs with horses (42 minutes) compared to other species (26 minutes). Across all programs, the total amount of AAI time was around 10 hours over the course of the study. The activities that filled this contact time were described in varying amounts of detail. Future research should report the use of an AAI manual, the key components and procedures followed with fidelity assessments, and animal/handler certifications and standards.
The large variability of AAI characteristics indicates that the practice is not yet standardized. Further investigation of AAI should enlist techniques to evaluate the dosing and trajectory of change over time, to determine the most efficacious combination of personnel, animal, and participant time that is optimal for both the participants and the animals. Synthesizing the current evidence base in this review therefore focused on the broad concept of AAI for autism, rather than the outcomes of a specific protocol. To achieve the second aim of the review, key elements of research methodology were reviewed to evaluate the quality of the evidence and potential risk of bias.
Evaluating the Evidence Base
The state of science on AAI for autism has improved in recent years. The most notable changes from studies between 1989–2012 (O’Haire, 2013) and 2012–2015 (current review) include larger sample sizes (≤ 42 in the previous review versus ≤ 164 in the current review), the use of control or comparison conditions (64% versus 75%) such as an active or attention control (7% versus 43%), standardized outcome measures (36% versus 82%), blinded raters (14% versus 21%), and physiological assessments (7% versus 18%). These improvements in research methodology have raised the rigor of the evidence base on AAI for autism; however, there are many areas that require further advances.
Multiple types of research design are necessary to move forward the field of autism intervention research (Mesibov & Shea, 2011). Small sample sizes are not necessarily a weakness; however, single-subject methodology must include multiple assessments per individual across conditions (Kazdin, 2011). Additionally, some small studies in the current sample enlisted designs that are more appropriate for larger samples (such as randomized trials), and thus were likely underpowered to detect significant differences between groups. Further, there appears to be large variability in outcomes across individuals, which requires either homogenous sample selection for single-subject designs or substantially large samples to evaluate individual variation characteristics. Some evidence suggests that children with autism are less interested in animals than their typically-developing peers (Grandgeorge et al., 2015), so identifying the characteristics of the interaction that are uniquely efficacious for this population, if any, is an important research agenda. Given that not all individuals with autism will benefit, it is important to begin to determine for whom AAI is beneficial and under what circumstances. Initial evidence suggests that children with autism who have verbal language skills engage more with animals than with their non-verbal peers (Grandgeorge et al., 2015). Identifying mediators and moderators of change relies on larger datasets to enable sufficient power to detect differences based on participant characteristics and other treatment factors.
There was a high risk of bias in many studies, which did not enlist blinded assessments to corroborate parent and teacher reports. Though these individuals would be intimately familiar with participants, they may be subject to expectancy biases or placebo effects. The use of multiple assessment sources, including blinded assessments, will increase the validity of findings in future AAI studies. A further way to enhance the validity is to use active or attention control conditions (Marino, 2012). Less than half (43%) of the studies in the current review enlisted these types of controls. Without them, changes may be due to extraneous factors such as the presence of something fun and engaging, which does not necessarily need to be an animal. To identify the animal as the active ingredient in AAI, stringent control or comparison conditions must be enlisted.
The strongest study to date was a statistically powered randomized clinical trial of AAI with horses, compared to an active control condition of barn activities with a life size horse replica (Gabriels, Zhaoxing, et al., 2015). This study enlisted blinded assessors in addition to parent reports on both observational and standardized survey instruments that are widely used in autism treatment evaluation research (e.g., ABC). The outcomes therefore cannot be attributed to selection biases in the sample (due to randomization), expectancy biases of informants or demand characteristics (due to blinded raters and a placebo condition), novelty (due to the presence of a novel life size horse replica in the control condition), or construct confounding (due to nearly identical procedures except for the presence of a live animal). A manualized treatment protocol and fidelity assessments were implemented. The results of this study provide evidence for this particular AAI protocol with horses as a “probably efficacious treatment” for autism, given that it has one study which meets the criteria for a “well-established treatment” but has not yet been replicated; these criteria include a good group-design experiment showing statistically significant superiority to a psychological placebo, implementation of a treatment manual with a specified population, reliable and valid outcome measures, and appropriate data analysis (Chambless et al., 1998). To validate it as a “well-established treatment” or evidence-based practice, another independent research team needs to conduct a high-quality randomized trial using the same manual, compared to an active control condition (Chambless et al., 1998; Reichow, Volkmar, & Cicchetti, 2008). Alternatively, a large series of at least nine single-subject studies from independent investigators should replicate the findings (Chambless et al., 1998).
For other AAIs, these standards to reach the status of an evidence-based practice can also be sought. Concurrently, it will be productive to conduct intervention development research to construct an evidence-based manual prior to pursuing evidence-based treatment status. Dismantling studies can be used to determine which components of the treatment are essential or most effective (e.g. Kazdin, 2007). For example, targeted studies could be enlisted to determine which activities or strategies with animals are most effective for children with autism (e.g., group versus individualized programs, mounted versus ground activities with horses, physical contact versus observation of animals). The rigid definition of manual can also be expanded to accommodate written explanations of principles and protocols that allow for individualized modifications (Mesibov & Shea, 2011). Understanding the mechanisms and components of the AAI will strengthen the development of evidence-based best practices that maximize positive outcomes for both human and animal participants.
Taken together, the evidence base on AAI for autism is strong enough to establish general proof of concept, but not cohesive enough to validate any specific protocol as an evidence-based treatment at this time. To achieve the third aim of this review, proof of concept outcomes were collated and synthesized to identify potential areas of change from AAI for autism.
Outcomes of AAI for Autism
The most commonly reported outcome was increased social interaction, identified in 22 studies by 19 research teams across 14 countries. This finding mirrors AAI research with other populations, where animals act as social facilitators and social supports for humans (e.g., McNicholas & Collis, 2000). The robustness of the effect was evidenced by multiple assessment types including blinded behavioral observation and standardized informant reports, with active control and comparison conditions. Given the preponderance of data on this domain, changes in social interaction are highlighted as the most promising potential outcome from AAI for autism.
All other outcomes were assessed at a substantially lower frequency across studies. Proof of concept is therefore limited in these domains, which yielded both positive and mixed results. Findings were unanimously positive across studies for increases in positive emotions (7 studies) and reductions in physiological indicators of stress (3 studies). They were predominantly positive across studies measuring increased language and communication (9 of 12 studies) and improved motor skills (5 of 6 studies, all with horses). Mixed results (half reporting significant change) were identified for problem behaviors (4 of 8 studies), autism diagnostic scores (2 of 4 studies), and parental stress (1 of 2 studies). Outcomes were predominantly non-significant across studies evaluating restricted and repetitive behaviors such as stereotypy (2 of 5 studies). No studies reported significant declines or harm in any area of functioning.
Discrepancies or mixed findings across studies may be due to a variety of factors related to the intervention itself (e.g. species, protocol, dosage, personnel training), measurement (e.g. different standardized assessments, different behavioral observation definitions, qualitative interpretation), or methodology (e.g. comparison to different control conditions or lack thereof, sample size and power). Given the large variability and heterogeneity across studies, it is premature to draw conclusions about true efficacy differences. To build upon on the findings of existing research and this review, further investigation is essential to validate areas of potential promise (i.e., positive emotions, stress, language/communication, and motor skills) and understand areas with mixed results or identify the conditions under which they may occur (i.e., problem behaviors, autism diagnostic scores, and parental stress). At this stage, it appears likely that restricted and repetitive behaviors in autism are not substantially improved via AAI.
In addition to the areas evaluated in the current evidence, there are many open questions that remain unexplored. There are several outcome domains that have not yet been assessed, such as executive function or theory of mind, which bear direct relevance to autism intervention and may be influenced by the hypothesized pathway of social reward motivation that is different to exposure to human family members or peers. A parallel line of research has begun to examine the effects of AAI for children with attention deficit hyperactivity disorder (ADHD), which is a highly comorbid disorder (Busch et al., 2016). A direct comparison of effects for autism and ADHD may provide insights into differential outcomes and arousal implications for these common neurodevelopmental disorders.
One efficient way to advance the science is for service dog providers and other AAI personnel to systematically collect data on their clients and outcomes during the waitlist and treatment periods. A review of existing practices indicated that service dog providers for autism are not currently assessing outcomes using standardized instruments (Butterly, Percy, & Ward, 2013). Even small additions to their application and monitoring process would vastly enhance our knowledge of and ability to predict successful animal-human pairings to maximize outcomes. Other areas where research is lacking include standardized reporting of adverse events and of critical importance, the assessment and protection of animal welfare.
Based on the existing evidence from 28 studies synthesized in this systematic review, the provision of AAI for autism should be viewed as a possibly efficacious enrichment activity for autism that may increase social interaction. The rationale for “possibly efficacious” is that some good studies showed the treatment to be efficacious, but none reported replicating the same treatment manual or protocol. Status as an efficacious complementary or integrative treatment hinges on further research to establish and test manualized AAI protocols. The same treatment manual must be used in multiple well-designed studies before a treatment can be deemed “well-established.” Thus the continued use of varied (or absent) program manuals hinders progress of the field towards meeting the criteria of an evidence-based practice. It is also important to maintain a realistic perspective and recognize that animals will not cure autism (Creagan, Bauer, Thomley, & Borg, 2015), but instead may offer a complementary and integrative approach to promote and enhance treatment outcomes.
Limitations
Though largely comprehensive, the results of this systematic review are subject to several limitations. First, the inclusion criteria were limited to only published, peer-reviewed journal articles. It is possible that dissertations, theses, or other unpublished work may have identified non-significant findings that remain unpublished due to their failure to support investigator hypotheses. This phenomenon is often referred to as the “file drawer” effect, whereby non-significant findings are hidden in a file drawer instead of disseminated for publication. The allegiance of treatment researchers to validating their practices makes this a relevant concern for the AAI field. Second, the limitation to English language studies may have precluded the inclusion of a larger sample of international research. Third, no restrictions were made with respect to methodological rigor. Weighting findings from weaker designs equally to those with stronger designs may bias the outcomes of the review. However, it is unclear whether this weighting would bolster or diminish the evidence base on AAI for autism, given the contradictory nature of weak studies as potentially underpowered with heterogeneous samples, or designed with minimally stringent controls. Finally, the relatively short time frame of this review (roughly 3.5 years) may not have been long enough to generate empirically strong data.
Conclusion
Research on AAI for autism is increasing in prevalence and methodological rigor. All identified studies focused on children, with no research on adults. The characteristics of AAI programs are varied, with horses as the most commonly researched species, followed by dogs. Across a heterogeneous group of studies, the most consistent finding was increased social interaction. Areas of potential promise requiring further investigation include positive emotions, stress, and language or communication. Ongoing study should focus on technique refinement, evidence-based manualization, the effects of individual differences, and safeguards for animal welfare. Current practices should be viewed as potentially promising enrichment interventions, rather than stand alone or complementary evidence-based treatments.
Acknowledgements
I gratefully acknowledge Alison Kirkham, Kerri Rodriguez, and Noémie Guérin (Purdue University) for their assistance with data collection for the systematic review process as well as Peggy McCardle, Jim Griffin, Layla Esposito (Eunice Kennedy Shriver National Institute for Child Health and Human Development) and Sandra McCune (WALTHAM® Centre for Pet Nutrition), for their thoughtful and constructive comments in review of this manuscript.
References
- *.Ajzenman HF, Standeven JW, & Shurtleff TL (2013). Effect of hippotherapy on motor control, adaptive behaviors, and participation in children with autism spectrum disorder: a pilot study. The American Journal Of Occupational Therapy: Official Publication Of The American Occupational Therapy Association, 67(6), 653–663. doi: 10.5014/ajot.2013.008383 [DOI] [PubMed] [Google Scholar]
- Barker SB, & Wolen AR (2008). The benefits of human-companion animal interaction: A review. Journal of Veterinary Medical Education , 35(4), 487–495. [DOI] [PubMed] [Google Scholar]
- Beetz A, Julius H, Turner D, & Kotrschal K (2012). Effects of social support by a dog on stress modulation in male children with insecure attachment. Frontiers in Psychology, 3, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini S (2006). The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 21(3), 138–145. doi: 10.1177/10883576060210030201 [DOI] [Google Scholar]
- *.Borgi M, Loliva D, Cerino S, Chiarotti F, Venerosi A, Bramini M,. . . Cirulli F (2015). Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. [DOI] [PubMed] [Google Scholar]
- *.Burgoyne L, Dowling L, Fitzgerald A, Connolly M, P Browne J, & Perry IJ (2014). Parents’ perspectives on the value of assistance dogs for children with autism spectrum disorder: a cross-sectional study. BMJ Open, 4(6), e004786–e004786. doi: 10.1136/bmjopen-2014-004786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C, Tucha L, Talarovicova A, Fuermaier ABM, Lewis-Evans B, & Tucha O (2016). Animal-Assisted Interventions for Children with Attention Deficit/Hyperactivity Disorder: A Theoretical Review and Consideration of Future Research Directions. Psychological Reports, 118(1), 292–331. doi: 10.1177/0033294115626633 [DOI] [PubMed] [Google Scholar]
- Butterly F, Percy C, & Ward G (2013). Brief report: Do service dog providers placing dogs with children with developmental disabilities use outcome measures and, if so, what are they? Journal of Autism and Developmental Disorders, 1–6. doi: 10.1007/s10803-013-1803-1 [DOI] [PubMed] [Google Scholar]
- *.Byström KM, & Persson CAL (2015). The meaning of companion animals for children and adolescents with autism: The parents’ perspective. Anthrozoos, 28(2), 263–275. doi: 10.2752/089279315X14219211661813 [DOI] [Google Scholar]
- *.Carlisle GK (2014). Pet dog ownership decisions for parents of children with autism spectrum disorder. Journal of Pediatric Nursing, 29(2), 114–123. doi: 10.1016/j.pedn.2013.09.005 [DOI] [PubMed] [Google Scholar]
- *.Carlisle GK (2015). The Social Skills and Attachment to Dogs of Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(5), 1137–1145. [DOI] [PubMed] [Google Scholar]
- Carter AS, Davis NO, Klin A, & Volkmar FR (2005). Social development in autism In Volkmar FR, Paul R, Klin A, & Cohen D (Eds.), Handbook of Autism and Pervasive Developmental Disorders: Vol. 1. Diagnosis, development, neurobiology, and behavior (3rd ed., Vol. 1, pp. 312–334). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Chambless DL, Baker MJ, Baucom DH, Beutler LE, Calhoun KS, Crits-Christoph P,. . . Haaga DAF (1998). Update on empirically validated therapies, II. The Clinical Psychologist, 51(1), 3–16. [Google Scholar]
- *.Chen CC, Crews D, Mundt S, & Ringenbach SDR (2015). Effects of equine interaction on EEG asymmetry in children with autism spectrum disorder: A pilot study. International Journal of Developmental Disabilities, 61(1), 56–59. doi: 10.1179/2047387714Y.0000000044 [DOI] [Google Scholar]
- Creagan ET, Bauer BA, Thomley BS, & Borg JM (2015). Animal-assisted therapy at Mayo Clinic: The time is now. Complementary Therapies in Clinical Practice, 21(2), 101–104. doi: 10.1016/j.ctcp.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Davis TN, Scalzo R, Butler E, Stauffer M, Farah YN, Perez S,. . . Coviello L (2015). Animal assisted interventions for children with autism spectrum disorder: A systematic review. Education and Training in Autism and Developmental Disabilities, 50(3), 316–329. doi: 10.1016/j.rasd.2010.04.002 [DOI] [Google Scholar]
- Fine AH & Beck AM (2015). Understanding our kinship with animals: Input for health care professionals interested in the human-animal bond In Fine AH (Ed.), Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions (4th ed., pp. 3–10). San Diego, CA: Elsevier Inc. [Google Scholar]
- Fine AH, Tedeschi P, & Elvolve E (2015). Forward thinking: The evolving field of human-animal interactions In Fine AH (Ed.), Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions (4th ed., pp. 21–36). San Diego, CA: Elsevier Inc. [Google Scholar]
- *.Funahashi A, Gruebler A, Aoki T, Kadone H, & Suzuki K (2014). Brief Report: The Smiles of a Child with Autism Spectrum Disorder during an Animal-Assisted Activity May Facilitate Social Positive Behaviors--Quantitative Analysis with Smile-Detecting Interface. Journal of Autism and Developmental Disorders, 44(3), 685–693. [DOI] [PubMed] [Google Scholar]
- *.Fung S.-c., & Leung A. S.-m. (2014). Pilot study investigating the role of therapy dogs in facilitating social interaction among children with autism. Journal of Contemporary Psychotherapy, 44(4), 253–262. doi: 10.1007/s10879-014-9274-z [DOI] [Google Scholar]
- *.Fung SC (2015). Increasing the Social Communication of a Boy With Autism Using Animal-assisted Play Therapy: A Case Report. Advances in Mind-Body Medicine, 29(3), 27–31. [PubMed] [Google Scholar]
- *.Gabriels RL, Zhaoxing P, DeChant B, Agnew JA, Brim N, & Mesibov G (2015). Randomized controlled trial of therapeutic horseback riding in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55(7), 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.García-Gómez A, Risco ML, Rubi JC, Guerrero E, & García-Peña IM (2014). Effects of a program of adapted therapeutic horse-riding in a group of autism spectrum disorder children. Electronic Journal of Research in Educational Psychology, 12(1), 107–128. doi: 10.14204/ejrep.32.13115 [DOI] [Google Scholar]
- *.Ghorban H, Sedigheh RD, Marzieh G, & Yaghoob G (2013). Effectiveness of Therapeutic Horseback Riding on Social Skills of Children with Autism Spectrum Disorder in Shiraz, Iran. Journal of Education and Learning,2(3), 79–84. [Google Scholar]
- Grandgeorge M, Bourreau Y, Alavi Z, Lemonnier E, Tordjman S, Deleau M, & Hausberger M (2015). Interest towards human, animal and object in children with autism spectrum disorders: an ethological approach at home. European Child & Adolescent Psychiatry, 24(1), 83–93. doi: 10.1007/s00787-014-0528-9 [DOI] [PubMed] [Google Scholar]
- *.Grandgeorge M, Tordjman S, Lazartigues A, Lemonnier E, Deleau M, & Hausberger M (2012. a). Does pet arrival trigger prosocial behaviors in individuals with autism? PLoS ONE, 7(8), e41739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin T, Fine AH, O’Haire ME, Carlisle GK, & Bowers CM (2015). The role of animals for individuals with autism spectrum disorder In Fine AH (Ed.), Handbook on animal-assisted therapy: Foundations and guidelines for animal-assisted interventions (4th ed., pp. 225–236). San Diego, CA: Academic Press. [Google Scholar]
- *.Grigore AA, & Rusu AS (2014). Interaction with a therapy dog enhances the effects of social story method in autistic children. Society & Animals: Journal of Human-Animal Studies, 22(3), 241–261. doi: 10.1163/15685306-12341326 [DOI] [Google Scholar]
- *.Holm MB, Baird JM, Kim YJ, Rajora KB, D’Silva D, Podolinsky L,. . . Minshew N (2014). Therapeutic Horseback Riding Outcomes of Parent-Identified Goals for Children with Autism Spectrum Disorder: An ABA’ Multiple Case Design Examining Dosing and Generalization to the Home and Community. Journal of Autism and Developmental Disorders, 44(4), 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Jenkins SR, & Reed FDD (2013). An Experimental Analysis of the Effects of Therapeutic Horseback Riding on the Behavior of Children with Autism. Research in Autism Spectrum Disorders, 7(6), 721–740. [Google Scholar]
- Jobe LE, & White SW (2007). Loneliness, social relationships, and a broader autism phenotype in college students. Personality and Individual Differences, 42(8), 1479–1489. [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–27. [DOI] [PubMed] [Google Scholar]
- Kazdin AE (2011). Single-case research designs: Methods for clinical and applied settings. Oxford University Press. [Google Scholar]
- *.Lanning BA, Baier ME, Matyastik, Ivey-hatz J, Krenek N, & Tubbs JD (2014). Effects of Equine Assisted Activities on Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 44(8), 1897–1907. doi: 10.5014/ajot.62.4.416. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA,. . . Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed.), 339, b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L (2012). Construct validity of animal-assisted therapy and activities: How important is the animal in AAT? Anthrozoos, 25(3), 139–151. doi: [DOI] [Google Scholar]
- McNicholas J, & Collis GM (2000). Dogs as catalysts for social interaction: Robustness of the effect. British Journal of Psychology, 91(1), 61–70. [DOI] [PubMed] [Google Scholar]
- *.MdYusof MSB, & Chia NKH (2012). Dolphin Encounter for Special Children (DESC) Program: Effectiveness of Dolphin-Assisted Therapy for Children with Autism. International Journal of Special Education, 27(3), 54–67. [Google Scholar]
- Mesibov GB, & Shea V (2011). Evidence-based practices and autism. Autism, 15(1), 114–133. doi: 10.1177/1362361309348070 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Clinical Research Ed.), 339, b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Z, Albright J, Herwick R, Viera A, & Souza M (2016, July). Analysis of methods to oversee the use of animals in animal-assisted intervention research. Paper presented at the 14th Triennial International Association of Human-Animal Interaction Organizations Conference, Paris, France. [Google Scholar]
- *.O’Haire ME (2010). Companion animals and human health: Benefits, challenges, and the road ahead. Journal of Veterinary Behavior: Clinical Applications and Research, 5(5), 226–234. doi: 10.1016/j.jveb.2010.02.002 [DOI] [Google Scholar]
- *.O’Haire ME (2013). Animal-assisted intervention for autism spectrum disorder: A systematic literature review. Journal of Autism and Developmental Disorders, 43(7), 1606–1622. doi: 10.1007/s10803-012-1707-5 [DOI] [PubMed] [Google Scholar]
- *.O’Haire ME, McKenzie SJ, Beck AM, & Slaughter V (2013). Social behaviors increase in children with autism in the presence of animals compared to toys. PLoS ONE, 8(2), e57010. doi: 10.1371/journal.pone.0057010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME, McKenzie SJ, Beck AM, & Slaughter V (2015). Animals may act as social buffers: Skin conductance arousal in children with autism spectrum disorder in a social context. Developmental Psychobiology, Advance online publication. doi: 10.1002/dev.21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME, McKenzie SJ, McCune S, & Slaughter V (2014). Effects of classroom animal-assisted activities on social functioning in children with autism spectrum disorder. Journal of Alternative and Complementary Medicine, 20(3), 162–168. doi: 10.1089/acm.2013.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palley LS, O’Rourke PP, & Niemi SM (2010). Mainstreaming animal-assisted therapy. ILAR Journal, 51(3), 199–207. [DOI] [PubMed] [Google Scholar]
- Polheber J, & Matchock R (2013). The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. Journal of Behavioral Medicine, 1–8. doi: 10.1007/s10865-013-9546-1 [DOI] [PubMed] [Google Scholar]
- Reichow B, Volkmar FR, & Cicchetti DV (2008). Development of the evaluative method for evaluating and determining evidence-based practices in autism. Journal of Autism and Developmental Disorders, 38(7), 1311–1319. doi: 10.1007/s10803-007-0517-7 [DOI] [PubMed] [Google Scholar]
- Rossbach KA, & Wilson JP (1992). Does a Dog’s Presence Make a Person Appear More Likable?: Two Studies. Anthrozoos, 5(1), 40–51. doi: 10.2752/089279392787011593 [DOI] [Google Scholar]
- *.Salgueiro E, Nunes L, Barros A, Maroco J, Salgueiro AI, & Dos Santos ME (2012). Effects of a dolphin interaction program on children with autism spectrum disorders: an exploratory research. BMC Research Notes, 5, 199–199. doi: 10.1186/1756-0500-5-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams MJ, Fortney EV, & Willenbring S (2006). Occupational therapy incorporating animals for children with autism: A pilot investigation. American Journal of Occupational Therapy, 60(3), 268–274. [DOI] [PubMed] [Google Scholar]
- Solomon O (2012). Doing, being and becoming: The sociality of children with autism in activities with therapy dogs and other people. Cambridge Anthropology, 30(1), 109. [Google Scholar]
- *.Steiner H, & Kertesz Z (2015). Effects of therapeutic horse riding on gait cycle parameters and some aspects of behavior of children with autism. Acta Physiologica Hungarica, 102(3), 324–335. doi: 10.1556/036.102.2015.3.10 [DOI] [PubMed] [Google Scholar]
- *.Stevenson K, Jarred S, Hinchcliffe V, & Roberts K (2015). Can a dog be used as a motivator to develop social interaction and engagement with teachers for students with autism? Support for Learning, 30(4), 341–363. doi: 10.1111/1467-9604.12105 [DOI] [Google Scholar]
- *.Tabares C, Vicente F, Sánchez S, Aparicio A, Alejo S, & Cubero J (2012). Quantification of hormonal changes by effects of hippotherapy in the autistic population. Neurochemical Journal, 6(4), 311–316. doi: 10.1134/S1819712412040125 [DOI] [Google Scholar]
- *.Ward SC, Whalon K, Rusnak K, Wendell K, & Paschall N (2013). The Association between Therapeutic Horseback Riding and the Social Communication and Sensory Reactions of Children with Autism. Journal of Autism and Developmental Disorders, 43(9), 2190–2198. [DOI] [PubMed] [Google Scholar]
- White SW, Keonig K, & Scahill L (2007). Social skills development in children with autism spectrum disorders: A review of the intervention research. Journal of Autism and Developmental Disorders, 37(10), 1858–1868. doi: 10.1007/s10803-006-0320-x [DOI] [PubMed] [Google Scholar]
- Whyte EM, Behrmann M, Minshew NJ, Garcia NV, & Scherf KS (2015). Animal, but not human, faces engage the distributed face network in adolescents with autism. Developmental Science, 19(2), 306–317. doi: doi: 10.1111/desc.12305 [DOI] [PubMed] [Google Scholar]
- Wood L, Giles-Corti B, & Bulsara M (2005). The pet connection: Pets as a conduit for social capital? Social Science & Medicine, 61(6), 1159–1173. doi: 10.1016/j.socscimed.2005.01.017 [DOI] [PubMed] [Google Scholar]
- *.Wright HF, Hall S, Hames A, Hardiman J, Mills R, & Mills DS (2015). Acquiring a Pet Dog Significantly Reduces Stress of Primary Carers for Children with Autism Spectrum Disorder: A Prospective Case Control Study. Journal of Autism and Developmental Disorders, 45(8), 2531–2540. doi: 10.1007/s10803-015-2418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


