Abstract
Background:
The safety of operator-directed sedation (ODS) in the pediatric/congenital cardiac catheterization laboratory has been questioned. To our knowledge, the relative safety of ODS vs. general anesthesia in these cases has not to date been critically evaluated.
Methods:
A single-center retrospective cohort study was performed to compare the relative safety, cost, and times of catheterization procedures performed with ODS and those performed with general anesthesia from a cardiac anesthesiologist (GA). The risk of adverse outcomes was compared using propensity-score adjusted models. Using the same propensity score, procedure times and relative charges were also compared.
Results:
Over the study period, 4,424 procedures in 2,547 patients were studied. Of these, 27% of cases were performed with ODS. ODS procedures were 70% diagnostic procedures, 17% device closure of PDA, 5%, balloon pulmonary valvuloplasty, and 3% pulmonary artery angioplasty. The risk of adverse event in adjusted models for ODS cases was significantly lower than in GA cases (OR: 0.66, 95% CI: 0.45 to 0.95, p=0.03). Total room time and case time were also significantly shorter (p<0.001). Professional (charge ratio: 0.88, p<0.001) and hospital (charge ratio: 0.84, p<0.001) charges for ODS cases were also lower than those for GA cases.
Conclusion:
The study demonstrates that clinical judgment can identify subjects in whom ODS is not associated with increased risk of adverse events. The use of ODS was associated with reduced case times and charges. In combination, these findings suggest that the selective use of ODS can allow for greater efficiency and higher value care without sacrificing safety.
Keywords: Pediatric cardiology, outcomes, cost, anesthesia
CONDENSED ABSTRACT
Operator directed procedural sedation (ODS) has been used historically in congenital catheterization laboratories, but its safety has been questioned recently. A retrospective single-center cohort study was performed to evaluate the relative safety of ODS vs. general anesthesia (GA) adjusting for confounding using propensity score adjustment. Use of ODS was associated with reduced risk of adverse events, along with shorter case times and lower charges relative to GA cases. These results suggest that clinical judgement can identify subjects in whom ODS is not associated with increased risk, and that judicious use of ODS is associated with reduced cost and procedural time.
INTRODUCTION
There is increasing attention regarding the use of procedural sedation. Recommendations regarding professionalization of “sedation physicians” and increasing utilization of anesthesiologists have been introduced in the hopes of optimizing patient safety during procedures. The pediatric and congenital catheterization laboratory (PCCL) is an environment in which procedural sedation without an anesthesiologist has been used historically. PCCL differ from both conventional operating rooms and “sedation suites” in several ways. Catheterizations typically involve less noxious stimuli than surgical procedures. At the same time, PCCL procedures are increasingly complex, and are performed in patients with a broad range of hemodynamic stability and co-morbidities. Determining which patients would benefit from general anesthesia (GA) over interventionalist-directed sedation (ODS), therefore, is an important issue.
In 2016, an expert panel with representatives from the Congenital Heart Disease Section of the Society for Cardiac Angiography and Intervention (CHD-SCAI), Society for Pediatric Anesthesia (SPA), and Congenital Cardiac Anesthesia Society (CCAS) proposed guidelines for the use of sedation and anesthesia during PCCL procedures(1). These recommendations discussed potential high-risk patient populations, aspects of intra-procedural anesthetic practice, and optimal systems practice. They also defined a minimum level of provider expertise for individual procedures based on the Catheterization Risk Score for Pediatrics (CRISP) score(2), a previously described, pre-procedural risk score. An important aspect of these recommendations is codifying which cases are “appropriate” to perform without an anesthesiologist.
Large multi-center series identify the risk of major adverse events associated with catheterization as between 10–11%(3, 4). Adverse events attributable to sedation/anesthesia were rare, but some progressed to a life-threatening severity(5). To our knowledge, no studies have evaluated the relative risk of procedures done using ODS and those performed with GA, while accounting for patient and procedure characteristics that influence both the choice of sedation strategy and outcomes.
A subset of diagnostic and interventional procedures has habitually been performed at our institution using ODS. A retrospective cohort study was performed to evaluate whether ODS was associated with a higher risk of adverse events (AE) compared to GA.
METHODS
Study Population:
We performed a single-center retrospective cohort study. Our Institutional Review Board approved the study. Subjects were identified by querying our institutional database. Data were extracted directly. Additional review of the electronic medical record was performed as necessary.
All elective and urgent catheterization cases performed from 1/1/2011–9/30/2017 were considered for inclusion. Only subjects between 30 days and 25 years of age were included, restricting the study population to cases for which both GA or ODS would be considered. Additional exclusion criteria were 1) combined catheterization and electrophysiology studies, 2) cases with initial ODS and that were converted to GA electively to complete the intervention, as they were not representative of either ODS or GA cases. Cases with initial ODS that were converted to GA because of an adverse event, hemodynamic instability, or inadequate sedation were included in the ODS cohort as an intention-to-treat analysis. Next, the subset of PCCL procedures in which the association between ODS or GA and outcome could be fairly compared was identified by identifying procedure types where ODS was used habitually (>5 cases over the study period). These procedure types were: endomyocardial biopsy after orthotopic heart transplant, pulmonary vasodilator drug studies, other diagnostic catheterizations, closure of patent ductus arteriosus, balloon pulmonary valvuloplasty, balloon aortic valvuloplasty, pulmonary artery angioplasty, balloon angioplasty of coarctation, right ventricle to pulmonary artery conduit balloon angioplasty, Fontan fenestration closure, and veno-venous collateral occlusion. The study cohort was then restricted to cases in which one or more of these procedures was performed. GA cases with multiple procedures that included other procedure types were excluded.
Study measures:
Demographic data, cardiac diagnosis, and pre-procedural risk factors were collected. Procedural information included case times, procedures performed, hemodynamic data, and adverse events. Definitions for diagnoses, procedures, and adverse events in the database are recorded using definitions from the IMProving Adult and Congential Treatment (IMPACT®) Registry(3, 4). Hospital and professional charges were extracted from our institution’s billing records. Recent SCAI-CHD/SPA/CCAS recommendations for appropriate application of anesthesia in the PCCL are based on CRISP score(1, 2), so it was calculated for each case.
At our institution, patients received one of three types of sedation/anesthesia care during their catheterization procedure: 1) GA provided by a cardiac anesthesiologist, 2) intravenous sedating agents or 3) local analgesia with subcutaneous lidocaine without other sedation. GA at our institution is provided solely by anesthesiologists with training in cardiac anesthesiology. They do not cross-cover procedures in non-cardiac patients. For the latter two strategies, a registered nurse under the supervision of the interventional cardiologist provides medications and monitors the patient. These strategies were pooled together as ODS since no anesthesiologist was present.
Statistical Analysis:
The primary exposure for this study was GA versus ODS. The primary outcome of interest was the occurrence of major adverse events (MAE): death within 30 days, cardiac arrest, new arrhythmia, new heart valve regurgitation, tamponade, air embolus, embolic stroke, device malposition, device embolization, airway events, initiation of dialysis, intubation due to patient instability, initiation of extracorporeal membrane oxygenation, initiation of ventricular assist device, bleeding event, unplanned surgery due to catheterization complication, vascular complication requiring treatment, repeat catheterization due to complication of catheterization, and “other” events. Reports for all cases with “other” adverse events were reviewed to ensure that the events were not more accurately classified by another category.
Two secondary outcomes were defined prior to analysis: 1) case times and 2) charges to provide information about differential resource utilization and efficiency of the two strategies. Case times analyzed were 1) room time (the period from patient entry until exited), 2) sheath time (time from first access to sheath removal), 3) exit time (time from hemostasis until dismissal), and 4) hemostasis time (sheath removal to application of site dressing(s)). These times were measured to determine 1) whether ODS was associated with reduced total case time and 2) at what point(s) these benefits accrued. Our hypothesis was that room, sheath, and exit times would be increased in GA cases, but that no difference would be seen in hemostasis times.
Descriptive statistics for the characteristics of both groups were calculated to evaluate for systematic differences between them. We anticipated that factors influencing the choice between ODS or GA would also influence our outcomes. To address potential confounding by indication, a propensity score was developed. Characteristics that were not evenly distributed between the two cohorts were included in a multivariable logistic regression model whose outcome was GA/ODS, which was used to calculate a propensity score for the choice between GA and ODS. Balance and overlap were evaluated (Supplementary Figure 1) and found to be reasonable.
We then calculated a logistic regression model for MAE adjusting for propensity score. This method to adjust for confounding (instead of recalculating a risk adjustment model) was chosen because the number of MAE was relatively small and simulation studies have demonstrated that propensity score adjustment is more robust under these conditions(6). Elective versus urgent status was not included in the initial propensity score because it is subjective.
For secondary outcomes, analogous models were calculated for case times and charges. Because both charges and case times are continuous outcomes that 1) are necessarily positive and 2) left skewed, these models were calculated using generalized linear models with a gamma frequency distribution and log link. No single strategy is universally accepted for this type of data, simulation studies have demonstrated that models using a gamma distribution are more robust than other strategies(7). This strategy has been used successfully in previous studies of cost in congenital cardiology(8–10).
To generate comparable charge data across the study period, several adjustments were performed. Charges were adjusted for inflation to United States year 2017 dollars using the consumer price index for medical care. To adjust for fluctuations in billing practice over time, the fiscal year of each case was included as a covariate. Our institution does not permit reporting of absolute costs or charges, so the data are reported as a ratio of charges between GA and ODS. To provide a concrete estimate of cost savings, an estimated unit hospital cost for each procedure-type was calculated using previously published standardized costs(11). A weighted average was calculated based on the frequency of procedures in the ODS cohort multiplied by the standardized cost. When there was not a perfect match for a procedure-type available, a best-approximation of cost was generated using the cost of a procedure with similar technical complexity.
As a secondary analysis, we sought to measure 1) the degree to which our historical practice conformed to recent consensus recommendations and 2) whether practice consistent with these recommendations was associated with improved outcomes. These recommendations state that it is appropriate to perform cases with CRISP<2 without an anesthesiologist but that cases with CRISP≥2 should be performed with an anesthesiologist (1). For analysis, the study population was divided by both sedation strategy and CRISP score and calculated ratios of observed to expected (O/E ratio) MAE for each of these four groups. It is important to note that the outcomes used in the CRISP model differ from those used in this study. Several of the events used are not included in CRISP, so bleeding and “other” events were not included in the observed events for this section of the analysis. In addition, it should be noted that for several event types, CRISP and our database have different definitions. The composite MAE reported described includes relatively minor events not included in the CRISP event rates. Reported rates of MAE and O/E ratios are inevitably over-estimates. This will inflate O/E ratios of GA and ODS cases uniformly and should not result in bias. We hypothesized that clinical review by our staff better discriminated cases in which ODS would be risk-neutral than the published algorithm. If this was correct, 1) the O/E ratio for cases with high CRISP scores performed with ODS would be less than that for cases with lower CRISP scores performed with ODS and 2) the O/E ratio for cases where GA with a low CRISP score would be greater than that for other subgroups, reflecting this group includes patients at higher risk than predicted by CRISP score. Comparisons of these standardized ratios is qualitative, but 95% confidence intervals were calculated for observed events and O/E ratios to provide a measure of uncertainty.
Because of concern that inpatients would be more likely to 1) receive GA and 2) incur additional inpatient charges, we performed a sensitivity analysis restricted to cases that were performed in outpatients. A second sensitivity analysis was performed restricting cases to those with a total hospital length of stay ≤2 days. The latter analysis addressed both the aforementioned concerns about inpatient costs and also determined if different sedation strategy led to longer inpatient observations.
Missing data were infrequent and were addressed by chart review, so no imputation was applied. The primary analyses were pre-specified, and other analyses should be considered exploratory. No formal adjustment for multiple comparisons was made. All data analysis was performed using Stata MP 13 (Statacorp, College Station, TX) and R version 3.4.2 (R Development Core Team, Vienna, Austria).
RESULTS
Study population:
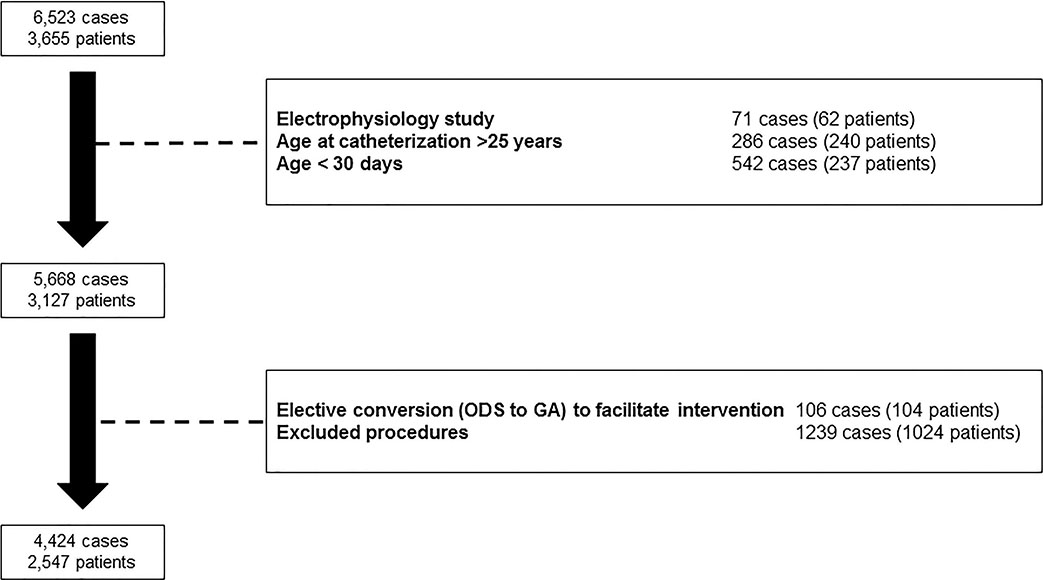
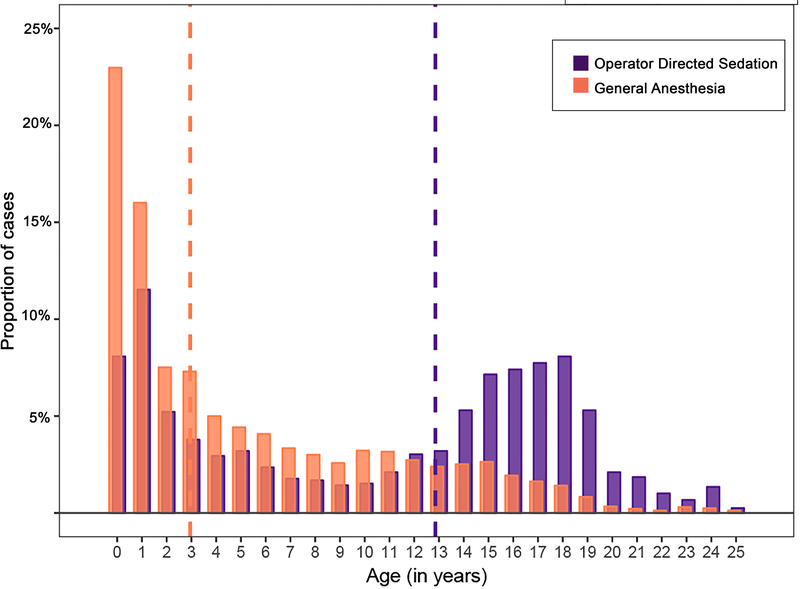
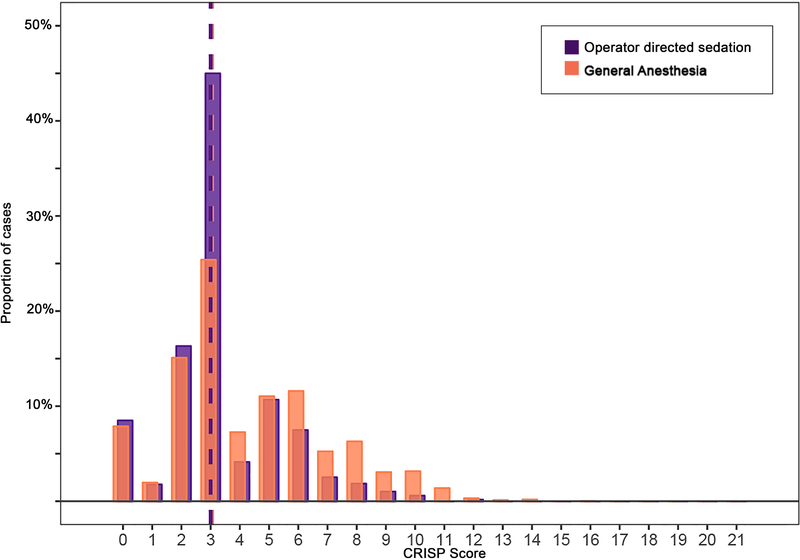
After applying exclusion criteria, the study population included 4,424 cases in 2,547 patients (Figure 1). Of these, 27% (n=1188) were performed using ODS. Specifically, 93% (1101/1188) were performed using IV sedation and 7% (88/1188) with local anesthetic alone. Characteristics of patients in GA and ODS cohorts are detailed in Table 1. The median age of subjects was lower in the GA cohort (p<0.001, Figure 2). In addition to age, cases with GA were performed in subjects with a higher proportion of prematurity, genetic syndromes, single ventricle heart disease, and chronic lung disease. These systematic differences are also reflected in the GA subgroup having a greater portion of higher CRISP scores (median: 3, IQR: 3–6) than the ODS subgroup (median: 3, IQR: 2–4, p<0.001, Figure 3).
Figure 1:
Study population
Table 1:
Study population
| General Anesthesia N=3236 | Physician Directed Sedation N=1188 | p | |
|---|---|---|---|
| Male sex | 1712 (53%) | 609 (51%) | 0.35 |
| Age at catheterization (years) | 3.0 (IQR: 0.6–9.0) | 12.8 (IQR: 2.6–16.9) | <0.001 |
| Age | |||
| 30 days – 1 year | 1079 (33%) | 182 (15%) | <0.001 |
| 1–8 years | 1254 (39%) | 287 (24%) | |
| 8–18 years | 809 (25%) | 517 (44%) | |
| 18–25 years | 94 (3%) | 202 (17%) | |
| Race | |||
| White | 1819 (56%) | 728 (61%) | <0.001 |
| Black | 633 (20%) | 256 (22%) | |
| Asian | 123 (4%) | 50 (4%) | |
| Other | 661 (20%) | 154 (13%) | |
| Premature infant | 500 (16%) | 73 (6%) | <0.001 |
| Genetic syndrome | 552 (17%) | 65 (5%) | <0.001 |
| Coagulation disorder | |||
| Hypocoagulation | 29 (0.9%) | 3 (0.3%) | 0.04 |
| Hypercoagulation | 43 (1.3%) | 5 (0.4%) | 0.02 |
| Weight (kg) | 13.1 (IQR: 6.7 to 26.1) | 40.2 (IQR: 12.5 to 63.0) | <0.001 |
| Single ventricle | 618 (19%) | 111 (9%) | <0.001 |
| Chronic lung disease | 456 (14%) | 44 (4%) | <0.001 |
| Renal and/or hepatic insufficiency | 7 (0.2%) | 2 (0.2%) | 1.00 |
| Pre-procedural inotrope | 47 (1.5%) | 1 (0.1%) | <0.001 |
| Status | <0.001 | ||
| Elective | 3094 (96%) | 1173 (99%) | |
| Urgent | 142 (4%) | 15 (1%) | |
| CRISP Score | 3 (IQR: 3–6) | 3 (IQR: 2–4) | <0.001 |
| CRISP Score ≥ 2 | 2917 (90%) | 1066 (90%) | 0.73 |
| Procedure type | |||
| Diagnostic catheterization | 1647 (51%) | 467 (39%) | <0.001 |
| Endomyocardial biopsy | 671 (21%) | 314 (26%) | |
| Pulmonary vasodilator drug study | 352 (11%) | 58 (5%) | |
| Device closure of patent ductus arteriosus | 107 (3%) | 199 (17%) | |
| Balloon aortic valvuloplasty | 39 (1%) | 21 (2%) | |
| Balloon pulmonary valvuloplasty | 51 (2%) | 61 (5%) | |
| Pulmonary artery balloon angioplasty | 224 (7%) | 33 (3%) | |
| Coarctation balloon angioplasty | 116 (4%) | 14 (1%) | |
| Conduit balloon angioplasty | 15 (0.5%) | 7 (0.6%) | |
| Device or coil occlusion of veno-venous collaterals and/or Fontan fenestration | 59 (2%) | 17 (1%) | |
| Multiple of the above interventions | 36 (1%) | 2 (0.2%) |
Abbreviations: IQR interquartile range
Figure 2: Study subject ages.
Median ages are depicted with dashed vertical lines. Operator directed sedation (purple) cases were performed in older subjects than general anesthesia (orange) cases (p<0.001).
Figure 3: CRISP scores in study subjects.
Medians are depicted with dashed vertical lines. CRISP scores were higher in the general anesthesia (orange) than in operator-directed sedation (purple) cases (p<0.001).
Risk of major adverse events:
The risk of all MAE in the population was 5.7% (n=253/4424) (Table 2). There were no in-hospital deaths. A total of 5 ODS cases (4.2%, 95%CI: 1.4–9.8%) were converted to GA because of MAE. GA cases had a higher rate of observed MAE (6.6%) than ODS cases (3.4%, p<0.001). Airway events occurred in 3 subjects (0.3%) receiving ODS, while none occurred in GA cases. No mortality was seen in either group.
Table 2:
Adverse events
| General Anesthesia N=3236 | Operator Directed Sedation N=1188 | p | |
|---|---|---|---|
| Total | 212 (6.6%) | 41 (3.5%) | <0.001 |
| 30 day in-hospital mortality | 0 (0%) | 0 (0%) | 1.00 |
| Cardiac arrest | 25 (0.8%) | 2 (0.2%) | 0.04 |
| New arrhythmia | 78 (2.4%) | 15 (1.3%) | 0.03 |
| New heart valve regurgitation | 2 (<0.1%) | 0 (0%) | 1.00 |
| Tamponade | 0 (0%) | 1 (<0.1%) | 0.60 |
| Air embolus | 0 (0%) | 0 (0%) | 1.00 |
| Embolic stroke | 3 (0.1%) | 0 (0%) | 0.69 |
| Device malposition | 1 (<0.1%) | 0 (0%) | 1.00 |
| Device embolization | 4 (0.1%) | 2 (0.2%) | 1.00 |
| Airway event | NA | 3 (0.3%) | NA |
| Initiation of dialysis | 1 (<0.1%) | 0 (0%) | 1.00 |
| New endotracheal intubation | 30 (0.9%) | 7 (0.6%) | 0.36 |
| Initiation of extracorporeal membrane oxygenation | 9 (0.3%) | 0 (0%) | 0.15 |
| Initiation of ventricular assist device | 3 (0.1%) | 1 (<0.1%) | 1.00 |
| Bleeding event | 32 (1.0%) | 11 (0.9%) | 0.99 |
| Unplanned cardiac/vascular/other surgery | 7 (0.2%) | 0 (0%) | 0.24 |
| Vascular complication | 43 (1.3%) | 4 (0.3%) | 0.01 |
| Repeat catheterization | 7 (0.2%) | 1 (<0.1%) | 0.60 |
| Other | 13 (0.4%) | 3 (0.3%) | 0.65 |
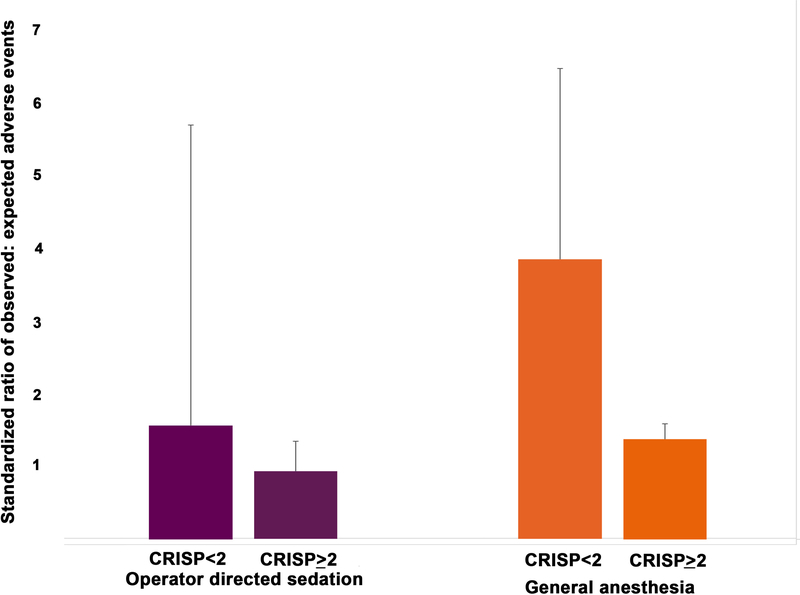
In propensity-score adjusted models, these differences remained significant. ODS was associated with reduced odds of MAE relative to GA (OR: 0.66, 95% CI: 0.45 to 0.95, p=0.03). In a pre-planned secondary analysis, we evaluated the degree to which our program has conformed to a recent SCAI-CHD/SPA/CCAS consensus document. Of ODS cases, the overwhelming majority fell outside of current recommendations; only 10.3% (122/1188) had CRISP scores (CRISP <2) that would have been “appropriate” to be performed without an anesthesiologist. According to these recommendations, 9.9% (319/3236) of cases performed with GA could have appropriately been performed with ODS (CRISP score <2). Given this discrepancy, we sought to evaluate whether the case-mix adjusted risk of adverse event (represented by O/E ratio for MAE) was different between cases where there was a deviation from guidelines (Figure 4). The point estimate for O/E ratio for ODS cases with higher CRISP scores (0.9, 95% CI: 0.6–1.3) was lower than that for ODS cases with CRISP<2 (1.6, 95% CI: 0.2–5.7) as well as those of high CRISP score cases performed with GA (1.4, 95% CI: 1.2–1.6). Conversely, GA cases in subjects with CRISP<2 had the highest O/E ratio (3.8, 95% CI: 2.1–6.5) of all four categories. The confidence intervals for O/E ratios are broad and, with the exception of the two sets of GA cases, overlap. At a minimum there is not a significant difference in the O/E ratio for cases with “appropriate” use of ODS cases and those higher CRISP scores.
Figure 4: Ratios of observed to expected outcomes.
Ratio of observed to expected outcomes (O/E ratio) for operator directed sedation (purple) and general anesthesia (orange) cases are depicted along with the top bound of 95% confidence intervals. Cases are further divided according to recent CHD-SCAI/SPA/CCAS recommendations.
Comparison of case times:
Propensity-score adjusted models were calculated to compare case times between ODS and GA cases (Central Illustration). ODS was associated with reduced total room time (ratio: 0.83, 95% CI 0.80 to 0.86, p<0.001) and sheath time (ratio: 0.87, 95% CI: 0.82 to 0.93, p<0.001) relative to GA. There was no significant difference in hemostasis time (ratio: 0.98, 95% CI: 0.92 to 1.04, p=0.47). ODS was also associated with shorter exit time than GA (ratio: 0.60, 95% CI: 0.54 to 0.68, p<0.001).
Central Illustration: Sedation strategy and case times.
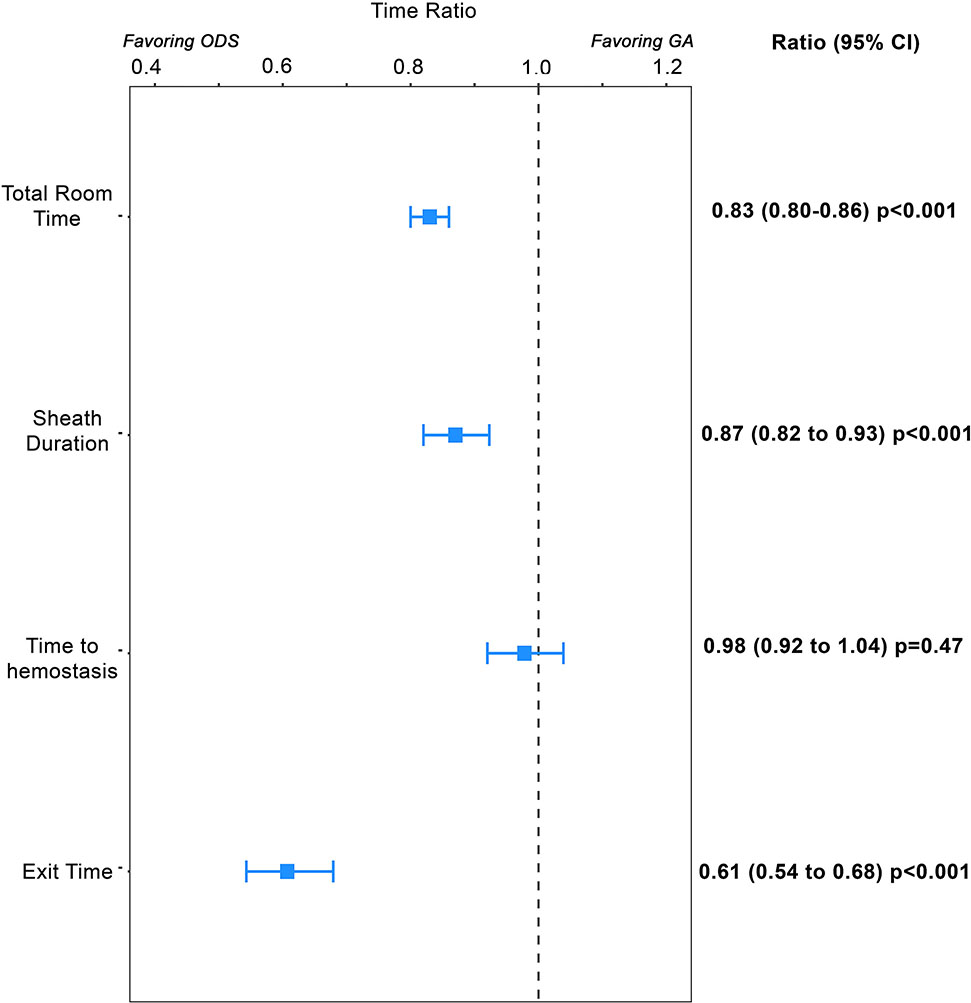
This Forest plot depicts the results of the propensity score adjusted generalized linear model for each of four different measurements of case time. The point estimate (box) of relative time for operator directed cases (ODS) vs. general anesthesia and 95% confidence intervals depicted (brackets) are depicted. Times for which both the point estimate and confidence intervals are to the left reflect times for which ODS cases are significantly shorter.
Comparison of hospital and professional charges:
Propensity score adjusted models were calculated to determine if sedation strategy was associated with significant differences in hospital and professional charges. ODS was associated with decreased hospital (ratio: 0.84, 95% CI: 0.82 to 0.87, p<0.001) and professional charges (ratio: 0.88, 95% CI: 0.85 to 0.92, p<0.001). Sensitivity analyses for outpatient catheterization and cases with total hospital length of stay ≤2 days did not differ significantly from the primary model (Supplementary Table 1). The weighted estimated average of the hospital cost for a single catheterization procedure with ODS was 2017US$13,985 and an estimated savings of 2017US$2,238 for each procedure performed using ODS. Using a conservative estimate that 20% of cases could be converted to ODS, the cost savings at a 500 case/year hospital would be 2017US$223,800. Savings from proportionally reduced professional costs would further increase these savings.
DISCUSSION
This single-center retrospective cohort study evaluated whether a program of ODS in a subset of PCCL procedures was associated with differences in MAE risk, case times, and economic impact compared to procedures performed with an anesthesiologist. In analyses adjusted for potential confounding factors, the risk of MAE was significantly lower in cases with ODS. We do not believe that this is evidence that treatment by an anesthesiologist increases the risk of a catheterization procedure or that these findings negate other aspects of the consensus statement. Rather, we propose that careful review of patient history can better identify patients in whom ODS is safe and effective and that its application can reduce resource utilization, specifically case times and costs.
A secondary analysis demonstrated that practice at our institution deviated from new guidelines (under which ~90% of ODS cases would be deemed inappropriate). This analysis demonstrated that deviations reflected judicious identification of cases with risks that were not consistent with their CRISP score. Individualized review of cases may be a better discriminator of risk with ODS. The value of identifying low risk patients for ODS emerges from the observation that ODS is associated with significantly lower charges and shorter case times (i.e. improved value).
The rationale behind standardization of sedation care in the PCCL is that involving a second provider with monitoring, airway management, and resuscitation skills can prevent adverse events. While this rationale is reasonable on face, there are a number of MAE for which the presence of an anesthesiologist would not have impact on risk of occurrence or management, reflecting potential limitation of methodologies using pooled MAE to determine which patients should receive care by an anesthesiologist. Recommendations defining appropriate/inappropriate practice in PCCL should be based on the best possible evidence. It is important to acknowledge uncertainty in data and allow latitude in these cases, especially where there may be benefits to the practice in question.
General anesthesia theoretically produces more hemodynamic stability during the procedure and less patient movement, both of which might facilitate safer and more rapid completion of procedures. Conversely, GA can also induce a state that is not representative of the patient’s physiology, obscuring important hemodynamic data. To meet regulatory requirements, our catheterization laboratory is revisiting our policies on ODS and is likely to utilize anesthesiologists for a greater percentage of cases moving forward. Current regulations call for a second qualified sedation practitioner to manage a patient when the goal of sedation is greater than moderate sedation (i.e. responsive to voice or light touch), and consensus recommendations mandate that the provider be credentialed to manage the next deeper level of sedation/anesthesia. The degree of sedation was not recorded in each case, and it is likely that some ODS patients achieved deeper levels of sedation. The current study is not able to evaluate whether either aspect of this recommendation improves safety. We are working with our anesthesia colleagues to identify situations in which care by an anesthesiologist does not necessitate endotracheal intubation and positive pressure ventilation and standardizing preparation for procedures to reduce some of the differences in case time we found in this study. We hope that these findings will help provide an evidence base to support continued excellent safety and procedural success, along with improved value in terms of both case times and cost.
ODS requires PCCL staffing with sufficient nurses/technologists to manage the technical aspects of the case while leaving a nurse free to provide sedation and monitoring. This might be challenging at centers with less flexibility in staffing. The benefits in terms of case times are also likely to be more valuable in higher volume centers, where catheterization room time may be at a premium and there is a demand to fill otherwise unused time. Maintaining excellent ODS requires both training and experience, which may hamper implementation at smaller-volume programs and those without a history of using ODS. Lastly, reducing the number of ODS cases at a single institution might counter-intuitively make these procedures less safe as team experience with ODS cases is sacrificed.
Identifying the proportion of major adverse events attributable to sedation practices in the ODS and GA subgroups would be useful. Adjudicating culpability for individual events was not possible in our study, so all MAE were studied without restriction. Counting how often adverse events were averted or rescued in both case types, but this was also not possible. Finally, we acknowledge that the definitions of AE in the CRISP methodology and in IMPACT® (the method used in our catheterization laboratory database) differ. The IMPACT® definitions include more minor events that would not be included in the CRISP model. The presented AE rates and O/E ratios are therefore inflated.
There are several other limitations to this study. The study population was limited to the practice at our institution, which limits generalizability, especially given the long institutional experience using ODS at our institution. In reports from the national IMProving Adult and Congenital Treatment Registry® (IMPACT®), >80% of procedures are performed with anesthesia(12), suggesting that our local practice differs significantly from a national sample of institutions. The results of this study are not, strictly speaking, generalizable beyond the studied procedures. However, the fact that some procedures were not done habitually with ODS does not imply that it is not possible/appropriate to do so. A multi-institutional study using data from a large clinical registry (e.g. IMPACT®) could identify the range of procedures performed with ODS in a broader range of centers and determine whether the results described are reproducible in that sample. This would also address potential type II error (especially important given how rare MAE are in this population). Though care was chosen in data collected and analysis, we also acknowledge the possibility of unmeasured confounding.
CONCLUSION
In a carefully selected group of patients and procedures, judicious use of ODS was associated with decreased charges, decreased case times without increased risk of MAE, delivering increased value without compromising safety.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Interventional cardiologist directed sedation has been used historically in pediatric and congenital cardiac catheterization laboratories (PCCL). Increasing regulatory scrutiny has been brought to bear on the use of non-anesthesiologist delivered sedation throughout pediatrics, and recent guidelines have outlined an algorithm guiding procedural sedation based on pre-procedural risk assessment. The population treated in PCCL procedures and the nature of PCCL procedures both make it a unique environment in terms of evaluating the safety of procedural sedation. Up to this point, however, issues with confounding by indication have made stringent evaluation of the relative safety of cardiologist directed sedation impossible.
WHAT IS NEW?
This study evaluates the safety of operator directed sedation (ODS) using propensity score adjustment to overcome confounding by indication. After adjustment, ODS cases were associated with reduced risk of major adverse events, lower procedural and hospital costs, and shorter case times relative to GA cases. Recent guidelines would recommend that 90% of ODS cases should have been performed with GA, though the ratios of observed to expected adverse events were not higher in ODS cases and GA cases regardless of CRISP score. This suggests that clinician judgment provided superior discrimination than the CRISP score in judging which patients might benefit from ODS, and that judicious use of ODS provides at least equal safety with reduced costs.
WHAT IS NEXT?
This study evaluated the experience at a single center, so expansion to the use of a multicenter dataset to evaluate the experience of other centers would be valuable. Regulatory bodies are in the process of restricting use of operator directed sedation and these decisions should be made (where possible) based on data.
ACKNOWLEDGEMENT
The authors would like to acknowledge Andrea Kennedy (The Children’s Hospital of Philadelphia’s Office of Clinical Quality Improvement) for performing queries of the Cardiac Center Clinical Database as well as Tyler Manning, Meaghan Lutts, and Evan Fieldston (The Children’s Hospital of Philadelphia) for their assistance in querying hospital billing data and insuring that the study is in compliance with our institution’s regulations regarding reporting of financial data. We would also like to acknowledge our catheterization laboratory staff (nurses and technologists) whose work is described in this manuscript.
Funding:
Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). The funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
ABBREVIATIONS AND ACRONYMS
- AE
adverse events
- CHD-SCAI/SPA/CCAS
Congenital Heart Disease section of the Society for Cardiac Angiography and Intervention, Society for Pediatric Anesthesia, and Congenital Cardiac Anesthesia Society
- CRISP
Catheterization Risk Score for Pediatrics
- GA
general anesthesia
- IMPACT®
IMProving Adult and Congenital Treatment registry
- IQR
interquartile range
- MAE
major adverse event
- ODS
operator directed sedation
- PCCL
pediatric congenital cardiac catheterization laboratory
Footnotes
Disclosures:
none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFEERENCES
- 1.Odegard KC, Vincent R, Baijal R, et al. SCAI/CCAS/SPA expert consensus statement for anesthesia and sedation practice: Recommendations for patients undergoing diagnostic and therapeutic procedures in the pediatric and congenital cardiac catheterization laboratory. Cathet. Cardiovasc. Intervent 2016;88:912–922. [DOI] [PubMed] [Google Scholar]
- 2.Nykanen DG, Forbes TJ, Du W, et al. CRISP: Catheterization RISk score for pediatrics: A Report from the Congenital Cardiac Interventional Study Consortium (CCISC). Cathet. Cardiovasc. Intervent 2015. [DOI] [PubMed] [Google Scholar]
- 3.Vincent RN, Moore J, Beekman RH, et al. Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiology in the Young 2016;26:70–78. [DOI] [PubMed] [Google Scholar]
- 4.Moore JW, Vincent RN, Beekman RH, et al. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. J Am Coll Cardiol 2014;64:2439–2451. [DOI] [PubMed] [Google Scholar]
- 5.Lin CH, Desai S, Nicolas R, et al. Sedation and Anesthesia in Pediatric and Congenital Cardiac Catheterization: A Prospective Multicenter Experience. Pediatr Cardiol 2015;36:1363–1375. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. AmJEpidemiol 2003;158:280–287. [DOI] [PubMed] [Google Scholar]
- 7.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ 2005;24:465–488. [DOI] [PubMed] [Google Scholar]
- 8.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Byrne ML, Shinohara RT, Grant EK, et al. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Byrne ML, Glatz AC, Mercer-Rosa L, et al. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol 2015;115:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Byrne ML, Shinohara RT, Mi L, et al. Inter-hospital Variation in Costs of Pediatric Cardiac Catheterization: An Analysis of the PHIS Database. Circ Cardiovasc Qual Outcomes 2018;11:A227. [Google Scholar]
- 12.Jayaram N, Spertus JA, O’Byrne ML, et al. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: A report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2017;183:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.